14-3-3 proteins are key regulatory factors in plants and are involved in a broad range of physiological processes. We focused on the evolutionary history of 14-3-3s from 46 angiosperm species, including basal angiosperm Amborella and major lineage of monocotyledons and eudicotyledons.
- 14-3-3 proteins
- whole-genome duplication
- coexpression
- gene family
- molecular phylogeny
- MrBayes
1. Introduction
2. Diversity of 14-3-3 isoforms in plants
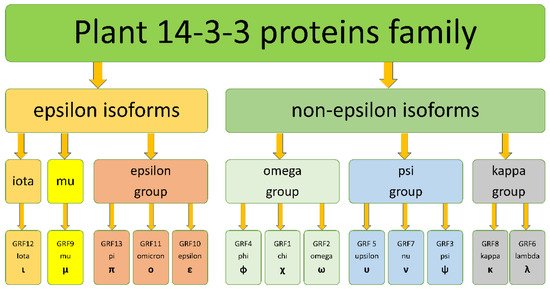
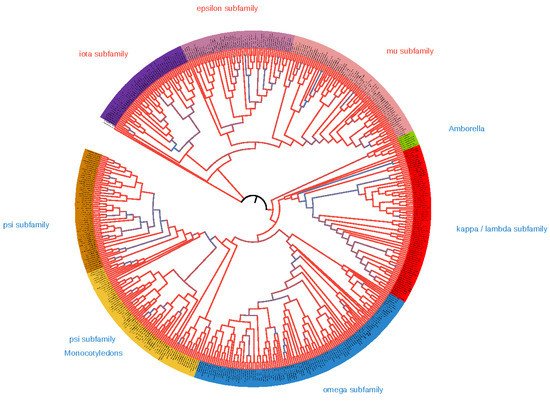
3. The origin of 14-3-3s diversity in Angiosperms
The diversity of isoforms of 14-3-3 proteins in plants is a result of gene duplication events. There were at least 106 whole-genome duplications (WGDs) in angiosperm evolution[19], and a lot of small-scale genome duplications (SSDs) in various plant lineages[20]. The early duplication event occurred in a common ancestor of green plants and lead to formation of pro-epsilon and pro-non-epsilon isoforms. Pro-epsilon probably was close to modern iota isoforms, whereas pro-non-epsilon is more similar with modern kappa isoforms. More late duplication events lead to the formation of iota and epsilon isoforms from pro-epsilon, and kappa and psi from pro-non-epsilon.
The origin of epsilon, pi, omicron, upsilon, nu, psi, chi, phi, omega, kappa and lambda 14-3-3 proteins in Arabidopsis, explained by two recent WGDs, took place in common ancestor of Brassicaceae family circa 14 and 80 million years ago [32,34,35]. At least three duplications produced high diversity of psi-group isoforms in Poaceae[21]. The vast variety of 14-3-3s in banana (25 isoforms)[17] could be driven by a combination of the WGD that happened 110–135 million years ago and three independent lineage-specific WGDs which happened 65–100 million years ago after the separation of banana’s ancestor from Poales[22].
Most of the 14-3-3 family gene duplications could be explained by WGD events, which happened many times during repeated cycles of “hybridization-polyploidization-secondary diploidization”, typical for plant evolution[23]. However, the variety of plant 14-3-3 proteins observed in angiosperms plants is explained not only by duplication, but also by lineage-specific gene loss. For example, the Poaceae family (grasses) lost mu and kappa isoforms, and all Poales (grasses and pineapple) lost the iota subfamily, and the Fabaceae family lost the epsilon-group isoforms. During the cycles of “hybridization-polyploidization-secondary diploidization”, polyploidization is often followed by loss of genetic materials, from individual genes to whole chromosomes.
4. Functionla groups of 14-3-3s vs. their evolutionary origin
14-3-3 proteins play a fundamental role in the regulation of plant physiology, thus there is a question of how species cope with a loss of whole 14-3-3 subfamilies. It is still unknown if 14-3-3 proteins are involved in physiological function in an isoform-specific manner. In an interactome study on developing A. thaliana’s seeds, 27 non-specific proteins were found to interact with 14-3-3s, 28 chi-specific (non-epsilon group) and 49 epsilon-specific[24]. Non-epsilon isoforms omega, kappa, lambda and chi better bind with plasma membrane H+-ATPase than epsilon, mu and omicron isoforms[25]. Different isoforms of 14-3-3 proteins show variation in binding activity with the C-terminus of plasma membrane H+-ATPase. Evolutionarily close phi and omega isoforms demonstrate an opposite capability for binding H+-ATPase[14]. The epsilon isoform in A. thaliana works as a positive regulator in cold-stress adaptation, lambda works as negative[26][27], but the epsilon-like isoform in rice is not affected by low-temperature stress [28].
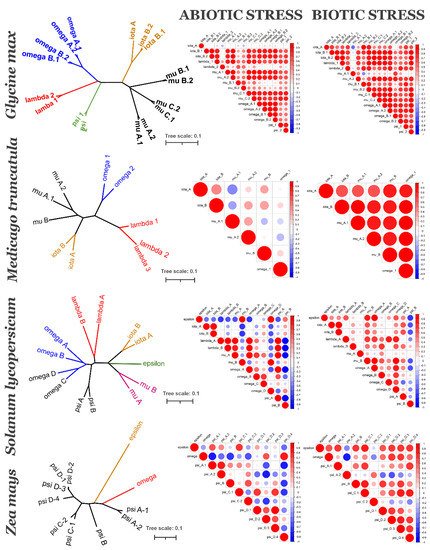
Figure 3. 14-3-3 isoforms of four species: Glycine max (soybean), Medicago truncatula (barrel medic), Solanum lycopersicum (tomato) and Zea mays (maize): phylogenetic relationships (left graph) and coexpression under abiotic and biotic stress (heatmaps). Left graph is unrooted NJ tree of 14-3-3s, bar is number of nucleotide changes per site. Colour of a branch reflects different subfamilies groups. Correlation heatmaps show PCCs between each pair of 14-3-3s. Positive correlations are displayed in red, negative correlations are displayed in blue. The size of the circle and the colour intensity are proportional to the PCCs.
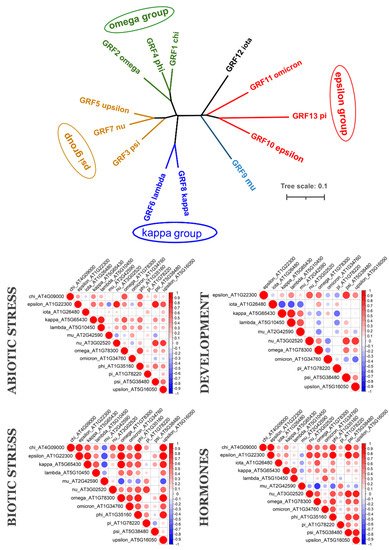
To understand if evolutionarily related 14-3-3 proteins are also functionally related, we analysed their coexpression using PLANEX and EXPath 2.0 databases. Coexpression data shows that each species has its own coexpression pattern for 14-3-3 proteins. The more diverse taxonomically they are, the more different functional groups formed 14-3-3 proteins (Figure 3). In A. thaliana (Figure 4), closely related isoforms from omega, psi and kappa groups had similar coexpression patterns under almost all physiological conditions. On the other hand, pi and omicron, which are closely related to epsilon and included in the epsilon group, showed negative correlation in expression with epsilon. In other species, closely related isoforms were often coexpressed, but not always (Figure 3). The suggestion is that lineage-specific gene loss (such as loss of epsilon in Fabaceae and loss of mu and iota in Poaceae) could be compensated by defunctionalisation of remaining isoforms.
This entry is adapted from the peer-reviewed paper 10.3390/plants10122724
References
- Michele K. Dougherty; Deborah K. Morrison; Unlocking the code of 14-3-3. Journal of Cell Science 2004, 117, 1875-1884, 10.1242/jcs.01171.
- Moore, B.; Perez, V.. Physiological and Biochemical Aspects of Nervous Integration; Prentice-Hall: Englewood: NJ, USA, 1967; pp. 343–359.
- G. Lu; A. J. DeLisle; N. C. de Vetten; R. J. Ferl; Brain proteins in plants: an Arabidopsis homolog to neurotransmitter pathway activators is part of a DNA binding complex.. Proceedings of the National Academy of Sciences 1992, 89, 11490-11494, 10.1073/pnas.89.23.11490.
- N C De Vetten; G Lu; R J Feri; A maize protein associated with the G-box binding complex has homology to brain regulatory proteins.. The Plant Cell 1992, 4, 1295-1307, 10.1105/tpc.4.10.1295.
- Stephan Hirsch; Alastair Aitken; Uwe Bertsch; Jürgen Soll; A plant homologue to mammalian brain 14-3-3 protein and protein kinase C inhibitor. FEBS Letters 1992, 296, 222-224, 10.1016/0014-5793(92)80384-s.
- Fiona C. Denison; Anna-Lisa Paul; Agata K. Zupanska; Robert J. Ferl; 14-3-3 proteins in plant physiology. Seminars in Cell & Developmental Biology 2011, 22, 720-727, 10.1016/j.semcdb.2011.08.006.
- Valérie Cotelle; Nathalie Leonhardt; 14-3-3 Proteins in Guard Cell Signaling. Frontiers in Plant Science 2016, 6, 1210, 10.3389/fpls.2015.01210.
- Albertus H. De Boer; Paula van Kleeff; Jing Gao; Plant 14-3-3 proteins as spiders in a web of phosphorylation. Protoplasma 2012, 250, 425-440, 10.1007/s00709-012-0437-z.
- Xing Zhao; Fanghui Li; Kunzhi Li; The 14‐3‐3 proteins: regulators of plant metabolism and stress responses. Plant Biology 2021, 23, 531-539, 10.1111/plb.13268.
- Jiaqi Liu; Shengliang Cao; Guofei Ding; Bin Wang; Yingchao Li; Yuzhong Zhao; Qingyuan Shao; Jian Feng; Sidang Liu; Liting Qin; et al. The role of 14‐3‐3 proteins in cell signalling pathways and virus infection. Journal of Cellular and Molecular Medicine 2021, 25, 4173-4182, 10.1111/jcmm.16490.
- Anna-Lisa Paul; Fiona C. Denison; Eric R. Schultz; Agata K. Zupanska; Robert J. Ferl; 14-3-3 phosphoprotein interaction networks – does isoform diversity present functional interaction specification?. Frontiers in Plant Science 2012, 3, 190, 10.3389/fpls.2012.00190.
- Alastair Aitken; D.B. Collinge; B.P.H. van Heusden; T. Isobe; P.H. Roseboom; G. Rosenfeld; J. Soll; 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends in Biochemical Sciences 1992, 17, 498-501, 10.1016/0968-0004(92)90339-b.
- Jernej Šribar; Nicholas E Sherman; Petra Prijatelj; Grazyna Faure; Franc Gubenšek; Jay W Fox; Alastair Aitken; Jože Pungerčar; Igor Križaj; The neurotoxic phospholipase A2 associates, through a non-phosphorylated binding motif, with 14-3-3 protein γ and ε isoforms. Biochemical and Biophysical Research Communications 2003, 302, 691-696, 10.1016/s0006-291x(03)00228-6.
- Magnus Rosenquist; Paul Sehnke; Robert J. Ferl; Marianne Sommarin; Christer Larsson; Evolution of the 14-3-3 Protein Family: Does the Large Number of Isoforms in Multicellular Organisms Reflect Functional Specificity?. Journal of Molecular Evolution 2000, 51, 446-458, 10.1007/s002390010107.
- Justin M. DeLille; Paul C. Sehnke; Robert J. Ferl; The Arabidopsis 14-3-3 Family of Signaling Regulators. Plant Physiology 2001, 126, 35-38, 10.1104/pp.126.1.35.
- Ken-Ichi Konagaya; Yasuhiko Matsushita; Masahiro Kasahara; Hiroshi Nyunoya; Members of 14-3-3 protein isoforms interacting with the resistance gene product N and the elicitor of Tobacco mosaic virus. Journal of General Plant Pathology 2004, 70, 221-231, 10.1007/s10327-003-0113-4.
- Meiying, Li; Licheng, Ren; Biyu, Xu; Xiaoliang, Yang; Qiyu ,Xia; Pingping, He; Susheng, Xiao; Anping, Guo; Wei, Hu; Zhiqiang, Jin; et al. Genome-Wide Identification, Phylogeny, and Expression Analyses of the 14-3-3 Family Reveal Their Involvement in the Development, Ripening, and Abiotic Stress Response in Banana. Frontiers in Plant Science 2016, 7, 1442, 10.3389/fpls.2016.01442.
- Hui Cao; Yuxing Xu; Linlin Yuan; Yanwei Bian; Lihui Wang; Shoumin Zhen; Yingkao Hu; Yueming Yan; Molecular Characterization of the 14-3-3 Gene Family in Brachypodium distachyon L. Reveals High Evolutionary Conservation and Diverse Responses to Abiotic Stresses. Frontiers in Plant Science 2016, 7, 1099, 10.3389/fpls.2016.01099.
- Jacob B. Landis; Douglas E. Soltis; Zheng Li; Hannah E. Marx; Michael S. Barker; David C. Tank; Pamela S. Soltis; Impact of whole‐genome duplication events on diversification rates in angiosperms. American Journal of Botany 2018, 105, 348-363, 10.1002/ajb2.1060.
- Lorenzo Carretero-Paulet; Mario A. Fares; Evolutionary Dynamics and Functional Specialization of Plant Paralogs Formed by Whole and Small-Scale Genome Duplications. Molecular Biology And Evolution 2012, 29, 3541-3551, 10.1093/molbev/mss162.
- Xin Qiao; Qionghou Li; Hao Yin; Kaijie Qi; Leiting Li; Runze Wang; Shaoling Zhang; Andrew H. Paterson; Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biology 2019, 20, 1-23, 10.1186/s13059-019-1650-2.
- Angélique D’Hont; France Denoeud; Jean-Marc Aury; Franc-Christophe Baurens; Françoise Carreel; et al.; The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213-217, 10.1038/nature11241.
- A. V. Rodionov; A. V. Amosova; E. A. Belyakov; P. M. Zhurbenko; Yu. V. Mikhailova; E. O. Punina; V. S. Shneyer; I. G. Loskutov; O. V. Muravenko; Genetic Consequences of Interspecific Hybridization, Its Role in Speciation and Phenotypic Diversity of Plants. Russian Journal of Genetics 2019, 55, 278-294, 10.1134/s1022795419030141.
- Kirby N. Swatek; Katherine Graham; Ganesh K. Agrawal; Jay J. Thelen; The 14-3-3 Isoforms Chi and Epsilon Differentially Bind Client Proteins from Developing Arabidopsis Seed. Journal of Proteome Research 2011, 10, 4076-4087, 10.1021/pr200263m.
- Roberta Pallucca; Sabina Visconti; Lorenzo Camoni; Giovanni Cesareni; Sonia Michaela Melino; Simona Panni; Paola Torreri; Patrizia Aducci; Specificity of ε and Non-ε Isoforms of Arabidopsis 14-3-3 Proteins Towards the H+-ATPase and Other Targets. PLoS ONE 2014, 9, e90764, 10.1371/journal.pone.0090764.
- Ziyan Liu; Yuxin Jia; Yanglin Ding; Yiting Shi; Zhen Li; Yan Guo; Zhizhong Gong; Shuhua Yang; Plasma Membrane CRPK1-Mediated Phosphorylation of 14-3-3 Proteins Induces Their Nuclear Import to Fine-Tune CBF Signaling during Cold Response. Molecular Cell 2017, 66, 117-128.e5, 10.1016/j.molcel.2017.02.016.
- Sabina Visconti; Chiara D’Ambrosio; Anna Fiorillo; Simona Arena; Carlo Muzi; Michela Zottini; Patrizia Aducci; Mauro Marra; Andrea Scaloni; Lorenzo Camoni; et al. Overexpression of 14-3-3 proteins enhances cold tolerance and increases levels of stress-responsive proteins of Arabidopsis plants. Plant Science 2019, 289, 110215, 10.1016/j.plantsci.2019.110215.
- Yuan Yao; Ying Du; Lin Jiang; Jin-Yuan Liu; Molecular Analysis and Expression Patterns of the 14-3-3 Gene Family from Oryza Sativa. BMB Reports 2007, 40, 349-357, 10.5483/bmbrep.2007.40.3.349.
