Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Geology
Iron silicide minerals (Fe-Si group) are found in terrestrial and solar system samples. These minerals tend to be more common in extraterrestrial rocks such as meteorites, and their existence in terrestrial rocks is limited due to a requirement of extremely reducing conditions to promote their formation. Such extremely reducing conditions can be found in fulgurites, which are glasses formed as cloud-to-ground lightning heats and fuses sand, soil, or rock.
- iron silicides
- fulgurites
1. An Overview on Fulgurites and Prebiotic Chemistry
Fulgurites are glassy rocks that are formed when an electric discharge flows through materials such as rock, soil, and sand [1]. The electric discharge in nature is lightning [2,3], but sometimes, a man-made powerline also can be an electric discharge source that produces a fulgurite [4,5]. As a fulgurite forms, a high-energy electric discharge (peak currents as much as 200 kA) quickly (about 100 μs) travels through sand, soil, and clay [6]. This tremendous electric current causes rapid melting of the target material and forms an amorphous, tubular glassy mixture, which traces the path the current traveled through the target.
Fulgurites are categorized as a type of pyrometamorphic natural glass [7,8,9], where pyrometamorphism is a form of low-pressure, high-temperature metamorphism. Pyrometamorphism is usually surficial, and may be analogous to impact metamorphism, especially given recent discoveries of shock quartz within fulgurites [10,11]. Fulgurites can be partitioned based on differing spark sources and different target minerals [1]: natural fulgurites are those fulgurites formed by lightning hitting materials such as soil, sand, and rock, whereas artificial fulgurites are formed by man-made electrical power structures or sources such as downed powerlines that discharge into natural materials. Anthropogenic fulgurites are formed by natural lightning that strikes artificial substances, such as asphalt or concrete. A fulgurite can be both artificial and anthropogenic if an artificial discharge travels through man-made target material. The major differences between lightning-formed fulgurites and electrical powerline-formed fulgurites are the power and total reaction time. Natural lightning provides a huge, powerful discharge of energy (109 J per flash) [12], extreme high temperatures (range of 104–105 K) [13] but limited duration (100 μs) [2,14]. On the contrary, man-made discharge sources have significantly less power than a lightning strike but may stay in contact with a target material for a much longer reaction time (hours). The net result is that it can be difficult to differentiate between artificial and natural sources as both form fulgurites, though glass morphology and composition can provide clues as to what energy source formed them [1].
Fulgurites form when a substantially powerful electrical current strikes and flows through a target mineral. The process requires the creation of an electric arc, which occurs when the electrical voltage surpasses the target material breakdown strength. This electrical arc provides a high-energy, high-temperature, and high-reduction environment that heats the target materials [1]. Furthermore, the high-energy, charged plasma generated by the electric current may heat and expand the tube channel, which may then transfer thermal and kinetic energy into the target material and its surrounding area [15]. The tube size and morphology of the fulgurite depend on the target mineral composition [3] and physical characteristics, as well as the energy and duration of the discharge event [1,15]. The mineralogy of fulgurites reflects their unusual formation conditions and is characterized by highly reduced phases such as iron silicide minerals and iron phosphides (schreibersite), as well as high-temperature minerals such as cristobalite and baddeleyite, and high-pressure minerals such as shocked quartz and cubic ZrO2 [10,11,16,17,18].
2. The Occurrences of Iron Silicides in Fulgurites
During fulgurite-forming lightning strikes, an extremely high-energy and high-temperature environment is generated over the course of a second (the lightning strike timescale), which persists for a few seconds to minutes after the initial strike and cessation of current (the timescale for heat dissipation). These uncommon conditions enable the reduction of oxide minerals into oxygen-poor compounds, such as phosphate minerals transforming into schreibersite (Fe, Ni)3P and phosphite [19,20,21]; silicate minerals changing into reduced iron silicides and elemental silicon [2,22,23,24,25]; and carbon sources reduced to carbide minerals [26,27,28].
The iron silicide minerals are like iron phosphides and are a group of rare minerals that formed in reducing environmental conditions. Major natural iron silicide minerals include gupeiite (Fe3Si) [29], suessite ((Fe, Ni)3Si) [30], hapkeite (Fe2Si) [31], xifengite (Fe5Si3) [28,29], naquite (FeSi) [32], linzhiite (FeSi2) [33] and luobusaite (Fe0.84Si2) [34]. Iron silicide minerals are common to a variety of fulgurites and can be found globally. Iron silicides in fulgurites may also be a possible source of the strange occurrences of Fe-Si that are found in unusual locations. We discuss these below, with the caveat that some of these occurrences are within fulgurites with artificial electric discharge sources, or with sources that are ambiguous (where details of the formation event were not recorded, or lost by the observers). Nonetheless, the occurrences of FeSi minerals within fulgurites spans both artificial and natural sources, and indicates the formation of these minerals is not a rare occurrence.
The first occurrence of silicides in fulgurites was reported by Essene and Fisher [2], who discussed the presence of opaque, metallic spherules inside the glassy matrix of the Winans Lake fulgurite, which was formed by a natural lightning strike, and which showed no evidence of mixing with the much more oxidized matrix glass. This occurrence showed that metal and oxide liquids emerged unmixed at the time of fulgurite formation. Therefore, it was reasoned that the reduced iron silicide minerals, such as FeSi, FeTiSi2, and Fe3Si7, were formed associated with the fulgurite when these two separate liquids cooled down. Additionally, Essene and Fisher [2] proposed that the reduction of silicates to silicides (and elemental silicon) was coupled to an oxidation of carbon (in the form of graphite, with its precursor likely being a tree root) inside the Winans Lake fulgurite, though the authors suggested oxidation of atmospheric nitrogen may have contributed to the reduction environment.
However, Sheffer [22] argued that this oxidation of carbon or nitrogen provides a fraction of the reducing power needed for the conversion of iron oxide and silica into metal silicides. Sheffer [22] and Roberts et al. [35] investigated several lightning-formed fulgurites and compared the glasses to their organic-poor, starting target materials. They demonstrated that in most fulgurites, iron is reduced compared to the original minerals (averaging 66% reduction of Fe3+ to Fe2+). Phases more reduced than Fe2+ were reported in two fulgurites in the study by Sheffer [22]. In one fulgurite from a sandstone in West Virginia, iron silicide minerals (FeSi, FeTiSi2, and FeSi2) [22,35,36] were identified. Sheffer [22] argued that reductants are not necessary for the formation of these reduced minerals, and instead, the separation of oxygen from oxides by an isentropic, high-temperature heat pulse is sufficient to contribute to the reduced mineralogy of fulgurites. Furthermore, a lightning strike shockwave that hits the target materials, and its prolongation can similarity contribute to the reducing environment of fulgurites. Target mineral grain size and density can directly influence the shock wave propagation and the reduction of fulgurites [35].
3. The Formation of Iron Silicide in Fulgurites
The formation of iron silicides is contingent on a reducing environment that provides the necessary conditions for formation of these minerals. Given the strongly oxidizing nature of most of Earth’s surface, such conditions are generally rare. The reduction of iron oxide to iron, silica to silicon, TiO2 to Ti, and the FeSi redox mineral buffer are shown as Figure 3.
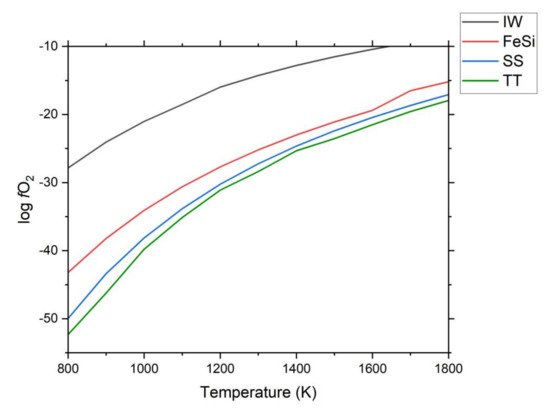
Figure 3. Oxygen fugacity mineral redox buffers for materials relevant to iron silicide formation. IW: Iron-wüstite; SS: Scheme 2; TT: Titanium-TiO2; FeSi: Iron-silicate to iron silicide (Fe + SiO2 = FeSi + O2). Data were collected from HSC software (version 9.3.0.9), Oxygen Fugacity Buffer Calculator (Australian National University, https://fo2.rses.anu.edu.au/fo2app/, accessed on 11 October 2021 and Hultgren et al. [45].
Generally, we can group iron silicide minerals in fulgurites into two major groups: silicon-rich and iron-rich. Silicon-rich iron silicide minerals are more commonly found in fulgurites, which in general are much richer in Si vs. Fe [2,19,22,25,38]. Iron-rich iron silicide minerals are more common in extraterrestrial materials, as iron metal is generally more abundant [30,46,47,48,49]. However, it is also plausible that fulgurites enriched in iron also contain iron-rich iron silicide [3], and silicon-rich iron silicides can be formed in the solar system [50] (Figure 4).
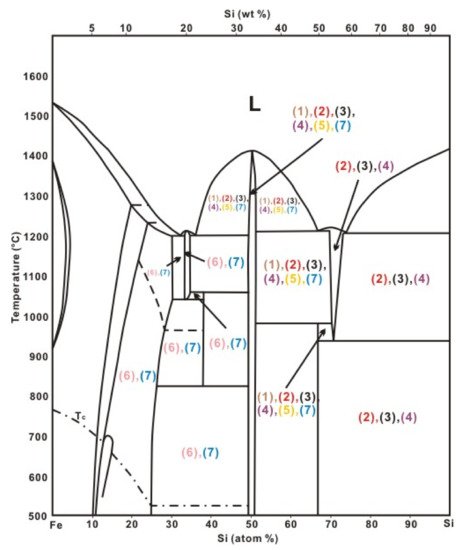
Figure 4. Iron-silicon phase diagram. The iron silicides associated with fulgurites are highlighted, which include (1) Winans Lake fulgurite (brown), (2) West Virginia fulgurite (red), (3) EI Rosario fulgurite (black), (4) Houghton Lake fulgurite (purple), (5) Sahara fulgurite (yellow), (6) York fulgurite (pink), and (7) Zacatecas fulgurite (blue). L represents liquid and Tc represents Curie temperature [2,3,22,25,37,38,41,51]. Modified after: Kubaschewski [51].
There have been several formation mechanisms proposed for iron silicide in fulgurites (Table 2). One plausible explanation is reduction with organic carbon. Carbon (such as cellulose from a tree root) can oxidize into carbon monoxide and carbon dioxide at high temperature, donating electrons to the surroundings. Thus, this interaction can reduce silicates and iron oxide into iron silicide [2,25,52]. Furthermore, it is possible that carbon reacts to form carbide with metal, creating the carbides Cr3C2, Cr2C, SiC, TaC, TiC, and WC [2], though these are not widespread in fulgurites. However, Sheffer [22] argues that this assumption is unlikely for all fulgurites due to the difference between the highly reducing environment required and stoichiometrically low amount of carbon available within most fulgurite target materials (e.g., sand fulgurites bear little to no carbon). However, in environments with heavy vegetation, iron reduction by a “smelting”-like process is likely the prevalent driver for silicide formation [3,22]. In the case of anthropogenic fulgurites, the presence of elemental aluminum (as the main material of some conducting powerlines) may serve as a reducing agent and drive silicide formation.
Table 2. Previous research for reduction mechanisms for forming iron silicides in fulgurites.
Alternatively, the galvanic reduction of target minerals could occur as the electrons flow from clouds to the ground during a lightning strike. This route has been considered with respect to iron silicide formation [2,53]. However, Sheffer [22] challenged this hypothesis, as the electron flows associated with lightning is usually tiny (~30 Coulombs [14]) and it would be difficult to quantitatively promote the reducing conditions necessary to form silicides and reduce iron (Fe2+).
Parnell et al. [41] state that high-temperature (>2000 K) liquid vapor deposition and rapid cooling could induce iron silicide formation. Reyes-Salas et al. [37] provide further evidence of vapor deposition in the microscopic morphology of the Zacatecas fulgurite. Pasek and Block [19] propose that at such high temperature, other reduction routes also become plausible, such as calcium phosphate reducing to calcium phosphite, which occurs in Type III fulgurites that typically do not have a significant inner void suggesting that vaporization is not intrinsically required for reduction, but may be important in silicide formation.
A shockwave associated with a lightning strike may also induce a reducing environment and support the formation of iron silicide [10,11,16,17,18,54]. However, Cardona et al. [25] argues that there is no evidence of shock that has been found in natural fulgurites, which was true at the time. Previous studies show that most fulgurites are characterized by the presence of α-quartz, which is not transformed into stishovite during a lightning strike, which would be expected if the high-temperature excursion was accompanied by high-pressure conditions [55]. Hence the shockwave associated with fulgurite formation may not be up to same level of power as a meteorite impact. Pasek et al. [3] provide the same conclusion that due to the lack of evidence for shock in York fulgurite, this shockwave reduction does not occur in the York fulgurite. However, recent findings suggest shock may be present in some fulgurites [10,11,17].
This entry is adapted from the peer-reviewed paper 10.3390/min11121394
This entry is offline, you can click here to edit this entry!
