You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agricultural Engineering
Scaffold-based bone tissue engineering has been introduced as an alternative treatment option for bone grafting due to limitations in the allograft. Not only physical conditions but also biological conditions such as gene expression significantly impact bone regeneration. Scaffolds in composition with bioactive molecules such as miRNA mimics provide a platform to enhance migration, proliferation, and differentiation of osteoprogenitor cells for bone regeneration.
- bone regeneration
- bone formation
- nanofiber
- miRNA delivery
- scaffold
1. Bone Healing and MiRNAs Expression
The adult bone adults constantly remodel itself, responding to both various physiological and pathological demands, such as repairing bones weakened by macro- and microdamage. Bone regeneration is a complex process, involving both bone remodeling and cellular processes. In both endochondral and intramembranous ossification, a number of complex gene expressions are involved in reaching the remodeled bone. Over the past several decades, our understanding of either process has improved enormously [12]. In both cases, MSCs migrate to the damaged area and increase growth factors and cytokines levels followed by differentiation into chondrocytes (endochondral ossification) or osteoblasts (intramembranous ossification). After an injury, a cartilaginous callus forms at the injury site, which provides intermediate stabilization to the fracture segment. This cartilage callus subsequently becomes vascularized, remodeled, and finally will be replaced by bone. Conversely, in intramembranous ossification, MSCs differentiate directly into osteoblasts in a highly stabilized fracture healing without a prolonged cartilaginous intermediate [13]. Fracture healing is an apparent example of endochondral ossification that begins with fracture-induced vascular disruption. Exposure of vascular cells triggers a coagulation cascade (thrombin activation) and the subsequent formation of hematoma, where platelets and macrophages accumulate. A local inflammatory reaction is initiated to release inflammatory mediators from the fracture hematoma, causing vascular permeability and inflammatory cell migration, as shown in Figure 2. These processes activate osteoclasts to resorb bone debris and fibroblasts to transform the hematoma into granulation tissue. New vasculature in the fracture site provides MSCs that transformed to the cells with osteogenic potential and formed callus. A soft and hard callus could convert to the bone via endochondral ossification and intramembranous ossification, respectively.
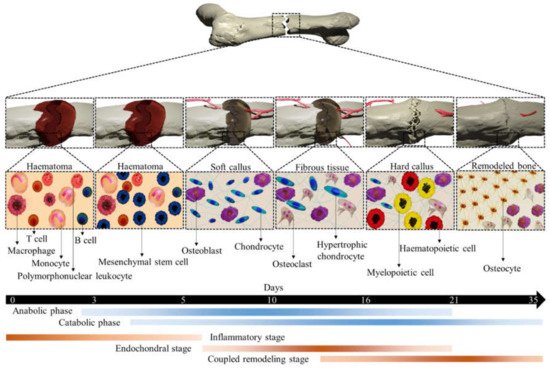
Figure 2. An illustration of different stages of typical fracture healing. The metabolic phases (blue bars) overlapped with biological stages (brown bars) for 35 days (black bars). The three biological stages of bone fracture healing are inflammatory, endochondral bone formation, and coupled remodeling. The time scale denoted is based on the primary cell types present at each stage of healing of a mouse closed femur fracture. This figure was recreated from 2D to 3D with permission from reference [14], Copyright 2021, Springer Nature.
Currently, more than 1000 articles in the PubMed database address questions about miRNAs in bone cells, including osteoblasts and osteoclasts. The microarray data from femoral fracture of twelve-week-old male Sprague-Daweley rats showed that 317 miRNAs were expressed more highly in normal healing fractures. However, eight miRNAs include rno-miR-140-3p, rno-miR-140-5p, rno-miR-181a-5p, rno-miR181d-5p, rno-miR- rno-208b-3p, rno-miR-451a, rno-miR-743b-5p, and rno-miR-879-3p, which were highly up-regulated by filtering with a fold change of >2.0, a low coefficient variation (<50%) during 28 days, as presented [15]. Recent studies demonstrated that miR-140-3p and miR-140-5p are highly expressed in the cartilage. Mmu-miR-140-3p and mmu-miR-140-5p are involved in osteoarthritis. Mmu-miR-140-3p with targeting the Cxcr4 and Rala promotes chondrogenesis and ameliorates osteoarthritis progression. Besides, the miR-140-5p with downregulating Il1b, Il6, Mmp13, Smad3, and Hmgb1 expression, inhibit inflammation. However, miR-140-5p with the upregulation of Dnpep, Tgfbr1, and Rala expression promotes chondrogenesis. Moreover, expression of miR-140-5p involves the upregulation of Hdac4 and Smad1, and results in the inhibition of chondrocyte hypertrophy and osteogenesis with downregulation Tlr4, Bmp2, and Tgfbr1 genes, respectively. MiR-181a-5p as well as miR-140-3p and miR-140-5p was identified as inflammation regulator at the early stage of fracture healing by targeting NCOA1, NRIP1, and IL1A gens. The treatment of human MSCs by miR-181d-5p mimic indicated that downregulation of miR-181d-5p promotes osteogenic differentiation by targeting RUNX2 through the MAPK pathway. Moreover, miR-181a-5p promotes osteoblastic differentiation via the procession of TGF-β secretion. Furthermore, both miR-181a-5p and miR-181d-5p, by upregulating BCL2, induce osteocyte apoptosis [16]. MiR-208b-3p was another miRNA that was expressed highly in bone fracture healing. Importantly, miR-208b-3p mimic suppressed osteoblast genesis in MC3T3-E1 and inhibited bone formation by reducing Acvr1b translation, thereby decreasing Bmp2 and its downstream target Smad1/4/5 and Runx2 [17].
Moreover, the overexpression of mmu-miR-451a could inhibit the osteogenic differentiation of mice MSCs, accelerate bone loss via Bmp6 signaling, and elevate bone loss by regulating Smad1/5/8 expression [18]. Additionally, miR-451a downregulates the CELF2, thereby significantly increasing COX protein and inhibiting chondrocyte hypertrophy and inflammation. It is unfortunate that still, there is no significant evidence for the role of miR-743b-5p and miR-879-3p in the bone field. Possibly, miR-879-3p is only expressed in mice and rats. However, it is reported that negative expression of mmu-miR-743b-5p could enhance fibrogenesis and Tgfb1 expression level [19]. TGFβ release from the bone matrix plays a vital role in the temporal and spatial regulation of bone remodeling during osteoclast bone resorption. Active TGFβ recruits MSCs to the bone resorption pit through the SMAD signaling pathway [20]. Moreover, activating the TGFβ pathway could promote angiogenesis in CRC cells [21]. The mi-RNAs involved in rat bone regeneration and considering their target genes applicable for human use are summarized in Figure 3.
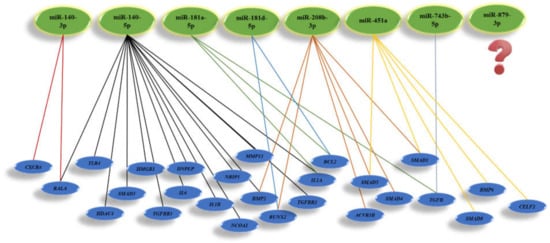
Figure 3. Illustration of miRNAs highly involved in bone regeneration and their target genes.
Intermembranous bone ossification, such as alveolar bone healing, is well studied by Anderia Espindola Vieira et al. [22]. It was found that intramembranous bone healing process in mice follows by tooth extraction and initiates with clot formation. Based on the results of the mice model, it appears that blood clots are gradually resorbed and replaced by granulation tissue associated with Col1a1 and Col1a2 expression over the course of 14 days. Moreover, they showed that growth factors and cytokines such as BMP2/4/7, FGF2, TGFβ1, VEGFA, TNFα, and IL10 were highly expressed during the first week of healing, indicating a lack of osteochondral gene markers. Unfortunately, up to now, there is no evidence related to miRNA expression in intramembranous ossification. The development of scaffolds may depend on understanding molecular signaling pathways involved in bone regeneration besides the miRNAs that are highly expressed during bone fracture healing.
MiRNA stability is a concern when considering clinical use; for example, many miRNAs rapidly degrade at 37 °C when incubated with serum [23]. MiRNA mimics are oligonucleotides that have been chemically altered to increase their stability and affinity for target genes [24,25]. Moreover, antagomirs, synthetic antagonists of miRNA, are currently undergoing phase 1 and phase 2 clinical trials. Therefore, various miRNA mimics and antagomirs have been evaluated for bone regeneration purposes. Few articles have reviewed miRNA incorporation with hydrogel scaffolds for bone regeneration [26], but none have focused on NFs. Among the trials, several miRNAs have been loaded to hydrogels for bone regeneration, as updated and summarized in Table 1.
2. Bone Tissue Regeneration by MiRNAs Mimic-NFs
Bone tissue engineering builds on the understanding of bone structure and aims to develop platforms that effectively regenerate bone defects. Bone derives its unique mechanical properties from an architectural design extending over nanoscale to macroscopic dimensions. A wide range of structural proteins are found in the ECM of bone, including serum-derived proteins, proteoglycans, glycosylated proteins, Gla-containing proteins, and small integrin-binding proteins. Mentioned proteins bind to strands of collagen fibrils (collagen type I, collagen type X, collagen type III, and collagen type V) [89], dominating at the nanometer level with a diameter scale between 35 to 60 nm. The hydroxyapatite crystals are embedded with collagen molecules and increase the rigidity of the bone. Besides, a wide range of proteins is engaged in the regulation of ECM formation [90]. Bone ECM also contains a number of biologically active molecules. For example, BMP2 [12], IGF1 [91], and TGF-β1 play crucial roles in osteoblast-osteoclast development and bone formation. RUNX2, a master transcriptional regulator of osteoblast differentiation [92], responds to numerous extracellular signals, including BMP and TGF-β1, and binds specific DNA sequences with several co-factors to regulate downstream target genes osteoblasts [93]. One such RUNX2 co-factor is SP7, and Sp subfamily member of the Sp/XKLF transcription factor family, leading to the upregulation of type I collagen [94]. SP7 also acts downstream of RUNX2 to regulate osteoblast development [36], exerting its regulatory effects by binding to guanine-rich sequences in specific target genes [95]. Several osteoblast-associated genes/gene products, including RUNX2, SP7, ALPL, COL1A1, IBSP, and BGLAP, are used as markers to demonstrate osteoblastogenesis in a developmental stage-dependent manner [96]. ALPL is a membrane-bound glycosylated enzyme that acts as a phosphatase to release inorganic phosphate from other molecules, increasing the concentration of phosphate required for calcification [97]. As of today, miRNA mimic-NF assemblies with osteogenic activity have been described only in a handful of publications (PubMed) summarizing cell culture models and laboratory animal models. James et al. provided the first report of the potential utility of a miRNA mimic-NF assembly [98]. In this example, electrospun and cross-linked gelatin nanofibers containing miRNA-29a inhibitors were supplied to MC3T3-E1 cells and mouse MSCs. Of the various target candidates tested, Tgfb1 and Igf1 were upregulated by the miRNA-29a inhibitor, leading to increased Sparc in the MC3T3-E1 cells and collagen deposition in MSCs. These NFs (35–50 µm thick) were stable for at least 7 days in the culture medium, and the release of miRNA-29a inhibitor into the medium was found as early as 2 h, gradually increasing and being sustained up to 72 h. To promote the differentiation switch toward osteoblast and accelerate the angiogenesis, osteogenic miR-22 and angiogenic miR-126 were introduced into electrospun PLC NFs [11]. In spite of their almost equal fiber diameter, the NFs derived from miR-22 and miR-26 had less mechanical strength and hydrophobicity. Cell adhesion, proliferation, and osteogenic differentiation of human iPS cells cultured under osteogenic conditions were enhanced by PLC NFs containing miR-22 and miR-26 compared to NFs without these miRNAs. In these cultures, more than 50% of the incorporated miRNAs were released during the first 72 h. Additionally, the 3D nanofiber aerogels containing miR-26a mimic were developed for the regeneration of calvaria bone defects in a rat model. The results of implantations indicate that aerogel- miR-26a mimic promotes bone formation up to 55%, while the aerogel loaded with negative control of miRNA could encourage bone formation up to 20%, as shown in Figure 6. Table 2 represents miRNA mimic-related molecules incorporated into NFs scaffolds for bone regeneration [99].
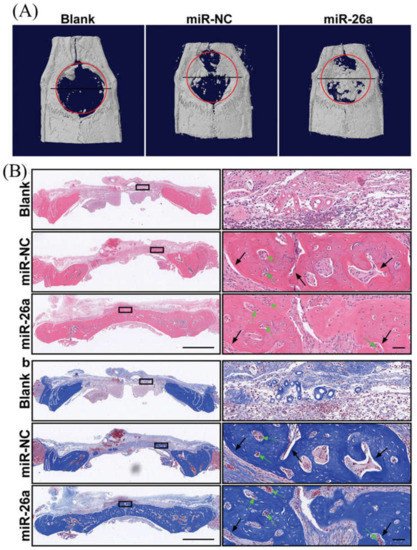
Figure 6. Representative planar radiographs of cranial bone defects at 4 weeks after implantation aerogel nanofibers contain negative control miRNA and miR-26a (A), H & E staining, and MTC staining of regenerated tissue after 4 weeks implantation of aerogel nanofibers include negative control miRNA and miR-26a (B). The figure is reprinted with permission from reference [99], Copyright 2021, John Wiley and Sons.
Table 2. miRNA mimic-related molecules incorporated into NFs.
| NF | miRNA | Target Gene(s) | Model/Application | Ref |
|---|---|---|---|---|
| Crosslinked gelatin | TransIT-TKO®-Antagomir-29a complex | Tgfb1, Igf1 | Mouse MC3T3E1 cells, mouse MSCs in vitro | [98] |
| PLC-gelatin NF | Polyplex-miR-22 and miR-26 mimic complex | RUNX2, SPARC, BGLAP | Human iPS cells in vitro | [11] |
| PLL-PHN-NF-SMS | HP-antagomir-199a complex | Hif1a | Rabbit MSCs in vitro, subcutaneous implantation in nude mice, implantation into an intervertebral disc degeneration rabbit model |
[100] |
| PLLA-NF-SMS | HP-miR-10a mimic complex | Irs1 | Mouse T cells, injection of complex plus TGFβ1 into a periodontitis mouse model |
[101] |
| PLGA | miR-2861 mimic | RUNX2 | Human iPS cells, in vitro | [104] |
| PEG/PLGA | miR-181a/b-1 | PTEN/PI3KK/AKT | Adipose-drived mesenchymal stem cells, in vitro | [105] |
| HA-SS-PGEA | miR-26a | Runx2, Alpl, Bglap, and Bsp | Rat MSCs, rat calvaria defect model | [99] |
Two reports have extended our understanding of the effects of miRNA-NFs assemblies on skeletal regeneration in vivo [100,101]. Poly (L-lactic acid) (PLLA)-graft-poly (hydroxyethyl methacrylate) was employed to prepare nanofibrous porous solid microspheres. These solid microspheres with an average diameter between 30 and 60 μM and approximately 15 μM in pore size supported proliferation and chondrogenic differentiation of rabbit MSCs. When rabbit MSCs precultured on solid microspheres carrying antagomir-199a with a hyperbranched polymer vector were implanted into the subcutis of nude mice, and needle puncture-induced lumbar disc degeneration of rabbits, increased chondrogenesis and intervertebral disc regeneration, respectively, were seen. These results were attributed to the antagomir targeting Hif1a, with the subsequent upregulation of Sox9 and other chondrocyte markers. The scaffold carrying hyperbranched polyplex-miR-10a mimic (known as a T regulatory cell [Treg] marker) complexes, in combination with the Treg recruitment factors IL2 and TGFβ (2 mg of microspheres containing 0.8 nmol miR-10a mimic, 1mg IL2, and 2 mg TGFβ protein), served as an injectable scaffold [101]. The nanofibrous scaffold was 60–90 nm in diameter, with multiple pores, and was designed to release IL2, TGFβ, and miR-10a mimic differentially for recruiting Tregs locally and promoting their differentiation effectively. miR-10a was expected to exert its effect on the PI3K-AKT-MTOR pathway by targeting IRS-1 and enhancing Treg differentiation. Since Tregs are a crucial regulator of the immune system in maintaining tolerance to self-antigens and suppressing the activities of other immune cells [102], nanofibrous microspheres carrying the active molecules as above were evaluated in a mouse model of periodontitis, a typical inflammatory disease that leads to gingival recession and alveolar bone defects [103]. Local injection of this assembly into the periodontal margin effectively inhibited the upregulation of gene expression of the primary cytokines of effector T cells in parallel with an increased ratio of Tregs/Th17 cells and concomitantly depressed osteoclastic bone resorption in the foci.
Furthermore, human iPSCs were transduced with miR-2861 mimic, and then the osteogenic differentiation of cells was investigated when cultured on electrospun PLGA nanofibers. Surprisingly, the results indicated that osteogenic functions including RUNX2, ALPL, BGLAP, and SPARK in iPSCs improved when the transfected cells were cultured on the NFs and compared to the tissue culture plate. All these indicate the impact of NFs on osteogenic function [104]. Adipose-derived mesenchymal stem cells culturing on miR-181a/b-1 mimic incorporated with PLGA NFs compared with the same cells cultured on the tissue culture plate. The results revealed that adipose-derived mesenchymal stem cells cultured on NFs containing miR-181a/b-1 mimic expressed significantly more Runx2 and Spark than cells cultured on a tissue culture plate [105].
This entry is adapted from the peer-reviewed paper 10.3390/mi12121472
This entry is offline, you can click here to edit this entry!
