Plants are often exposed to unfavorable environmental conditions, for instance abiotic stresses, which dramatically alter distribution of plant species among ecological niches and limit the yields of crop species. Among these, drought stress is one of the most impacting factors which alter seriously the plant physiology, finally leading to the decline of the crop productivity. Drought stress causes in plants a set of morpho-anatomical, physiological and biochemical changes, mainly addressed to limit the loss of water by transpiration with the attempt to increase the plant water use efficiency. The stomata closure, one of the first consistent reactions observed under drought, results in a series of consequent physiological/biochemical adjustments aimed at balancing the photosynthetic process as well as at enhancing the plant defense barriers against drought-promoted stress (e.g., stimulation of antioxidant systems, accumulation of osmolytes and stimulation of aquaporin synthesis), all representing an attempt by the plant to overcome the unfavorable period of limited water availability. In view of the severe changes in water availability imposed by climate change factors and considering the increasing human population, it is therefore of outmost importance to highlight: (i) how plants react to drought; (ii) the mechanisms of tolerance exhibited by some species/cultivars; and (iii) the techniques aimed at increasing the tolerance of crop species against limited water availability. All these aspects are necessary to respond to the continuously increasing demand for food, which unfortunately parallels the loss of arable land due to changes in rainfall dynamics and prolonged period of drought provoked by climate change factors. This review summarizes the most updated findings on the impact of drought stress on plant morphological, biochemical and physiological features and highlights plant mechanisms of tolerance which could be exploited to increase the plant capability to survive under limited water availability. In addition, possible applicative strategies to help the plant in counteracting unfavorable drought periods are also discussed.
- Plant-Metabolism
- Drought
1. Influence of Drought Stress on Plant Performances: From Morpho-Anatomy to Biochemical Changes
Water deficit conditions stimulate several plant responses, such as morphological, physiological, biochemical and molecular alterations, which ultimately result in disturbing plant functioning [1] (Figure 1). As depicted in Figure 1, drought events limit plant performances in different developmental stages. Limited water availability can indeed reduce the germination rate and the development of young plants [2]. During the progression of plant growth, drought basically influences the plant water relations, which in turn cause severe perturbation to the whole plant metabolism (at physiological, biochemical and molecular levels), depending to the stress severity and duration [3]. Water deficit conditions alter several activities of plant, but one of the main effects is the decline of photosynthetic activity [4][5] and finally the plant yield [6][7]. During drought stress conditions, oxidative stress, directly or indirectly generated in plants, is one of the main drivers of plant responses and results in damage to cell membrane, altering membrane integrity, physiological and biochemical alterations which lead to acute metabolic disorders and eventually alter the plant productivity [8][9].
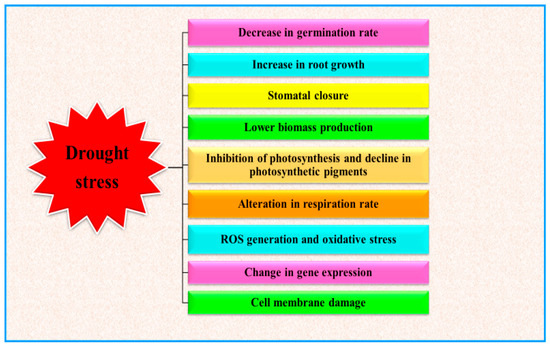
2. Drought Stress and Plant Growth
Drought stress is well recognized as a limiting factor which alters multiple aspects of plant growth and development. Germination of seeds, health and coleoptile length are foremost for the plant progression [11]. Seed germination is the primary aspect of growth which is sensitive to drought stress. Noteworthy alterations are observed in the seed germination of a plethora of plant species, including some of the most widely cultivated crops such as maize [12], sorghum [13] and wheat [14].
Visible symptoms of plant exposed to water scarcity in the initial vegetative stage are leaf wilting, decline in plant height and interruption in establishment of buds and flowers [15]. Drought conditions also limit the uptake of nutrients by the plants due to limited soil moisture, leading to decreased stem length [16]. Shoot length was also reduced under water deficit conditions in Lathyrus sativus L. [17]. In conditions of water deficit, plants seek to extract water from deeper soil layers by boosting their root architecture [18]. Moreover, water availability is primarily recognized by roots, which in turn regulates its growth and organization characteristics such as root length, spread, number and length of lateral roots [19]. Roots are crucial for different biological activities and plant yield, for instance nutrient accumulation and water absorption, and they are also involved in rhizosphere symbiotic associations with other microorganisms. Drought stress escalated root length in Crocus sativus L. [20]. Thus, a healthy root apparatus provides the benefit for sustenance of the escalation of plant growth, especially in the course of primary plant growth phase [21]. Escalation in root length is recognized as a useful strategy to increase soil water retention and nutrient accumulation to enhance plant biomass production [22]. Under water deficit, the plant root to shoot proportion generally improves, and, subsequently, the plant biomass decreases substantially [23].
The leaf is the chief part of the plant where most of the photosynthetic products are synthetized. The number of leaves decreased when subjected to water stress in Andrographis paniculate [24]. Optimal leaf development and the maintenance of an adequate leaf area is vital for photosynthesis, which in turn is the main driver of plant growth. Water stress causes reduction in leaf area, which results in decreased photosynthesis, hence reducing the crop yield. Leaf area declined under water stress conditions in Petroselinum crispum L. and in Stevia rabaudiana plants to achieve stability among the water absorbed by roots and the water status of various plant parts [25][26]. Reduction in leaf area is a drought avoidance strategy because declining leaf area results in a decreased water loss by the process of transpiration and this reduction in leaf area is attributable to the inhibition of leaf expansion by declined rate of cell division, which results in loss of cell turgidity [27]. Decrease in soil moisture causes a parallel reduction of leaf water content, which, in turn, induces a decline of turgor pressure of guard cells due to stomata closure [28]. Of note, the rate of premature leaf senescence is enhanced in drought environments .
3. Drought Stress and Photosynthesis
Major consequence of water deficit in plants is the decrease or suppression of photosynthesis [29](Figure 2). Reduced leaf area, increased stomata closure and consequent reduced leaf cooling by evapotranspiration increases osmotic stress leading to damages to the photosynthetic apparatus are among the major constraints for photosynthesis [30][31]. Among these, the decrease in photosynthetic process in plants under drought is mainly attributable to the decline in CO2 conductance via stomata and mesophyll limitations [32]. Decrease in photosynthetic activity due to drought may also be due to reduced ability of stomatal movement [33][34]. Declined activity of photosynthesis is triggered by the loss of CO2 [35] uptake, whose drop has been shown to affect Rubisco activity and decrease the function of nitrate reductase and sucrose phosphate synthase and the ability for ribulose bisphosphate (RuBP) production. Supportively, CO2 enrichment eliminated many early responses of maize metabolites and transcripts attributable to drought stress [36].
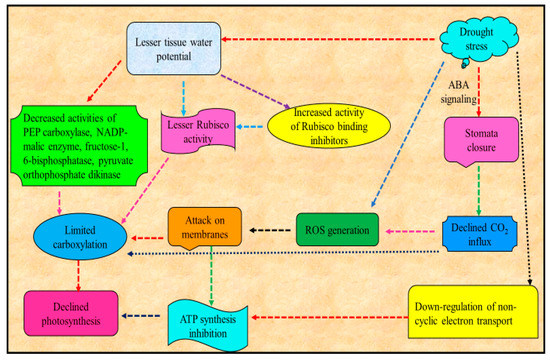
Figure 2. Schematic representation of effect of drought stress on photosynthesis .
Water deficit also resulted in decreased leaf area per shoot, and, thus, modification in canopy architecture, and this feature can alter gas exchange, water relations, vegetative growth and sink development (e.g., fruits or grains) [37], altering, for example, berry sugar concentration in grape [38] and biomass partition in maize (i.e., kernel number and 100-kernel dry weight decreased with increasing water stress duration) [39].
Chlorophyll content, which is of outmost importance for photosynthesis [40], is another photosynthetic attribute strongly influenced by water deficit that has been recognized as a distinctive indication of photo oxidation and degradation of chlorophylls [41]. For example, leaf chlorophyll synthesis and chlorophyll a/b proportion in soybean is altered by drought stress [42]. Decline in photosynthetic activity, amount of chlorophylls, loss of photosystem II photochemical efficiency, alteration in stomatal movement and disturbance in water status of plants resulted in declined plant productivity [43]. Among others, a major cause for decline in amount of chlorophyll due to drought stress is the drought-promoted O2− and H2O2, which results in lipid peroxidation and ultimately chlorophyll degradation [44]. The decrease of plant development and yield in several plant species under water deficit is often associated with decline in photosynthetic action and chlorophyll content impairment [45]. Water deficit alters the action of photosynthetic moieties and chlorophyll pigments, which ultimately resulted in reduced photosynthetic activities in Vigna mungo [46].
Drought stress induces a decreased net photosynthesis and also changes the plant carbon allocation and metabolism, which ultimately results in energy dissipation and declined yield [47]. For example, drought stress decreased the physiological metabolic disorders by suppressing the photosynthetic products production and disrupting the carbon balance in soybean. Drought stress also caused a reduction in the abundance of several Calvin cycle proteins, including Rubisco downregulation in olive [48]. Acute drought stress conditions also cause the damage to Rubisco enzyme and other enzymes associated with photosynthesis and are responsible for the loss of photosynthetic pigment content [49].
4. Drought Stress and Antioxidant Defense System
5. Drought Stress and Secondary Metabolites
6. Drought Stress and Mineral Nutrition
This entry is adapted from the peer-reviewed paper 10.3390/app10165692
References
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relationsand photosynthesis. Emir J. Food Agr. 2012, 24,57–72.
- Yigit, N.; Sevik, H.; Cetin, M.; Kaya, N. Determination of the effect of drought stress on the seed germination in some plant species. In Water stress in plants, Intech Open, London, UK, 2016, pp 43–62.
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86.
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681.
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas‐Carbó, M. Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol. Planta. 2006, 127, 343–352.
- Vurayai, R.; Emongor, V.; Moseki, B. Effect of water stress imposed at different growth and development stages on morphological traits and yield of bambara groundnuts (Vigna subterranea L. Verdc). Am. J. Plant Physiol. 2011, 6, 17–27.
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aus. J. Crop Sci. 2010, 4, 580.
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543.
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: physiological and molecular aspects. Plants 2019, 8, 190.
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal Crop Proteomics: Systemic Analysis of Crop Drought Stress Responses Towards Marker-Assisted Selection Breeding. Front Plant Sci 2017, 8, 757, doi:10.3389/fpls.2017.00757.
- Sourour, A.; Afef, O.; Mounir, R.; Mongi, B.Y. A review: morphological, physiological, biochemical and molecular plant responses to water deficit stress. Int. J. Eng. Sci. 2017, 6, 1–4.
- Queiroz, M.S.; Oliveira, C.E.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Menis, V.S.; Mello, B.F.F.R.; Cabral, R.C.; Menis, T.F. Drought stresses on seed germination and early growth of maize and sorghum. J. Agri. Sci. 2019, 11, 310–318.
- Patanè, C.; Saita, A.; Sortino, O. Comparative effects of salt and water stress on seed germination and early embryo growth in two cultivars of sweet sorghum. J. Agron. Crop Sci. 2013, 199, 30–37.
- Qayyum, A.; Razzaq, A.; Ahmad, M.; Jenks, M.A. Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat (Triticum aestivum L.) genotypes. Af. J.Biotech. 2011, 10, 14038–14045.
- Bhatt, R.M.; Rao, N.K.S. Influence of pod load on response of okra to water stress. Indian J. Plant Physiol. 2005, 10, 54–59.
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 2008, 10, 451–454.
- Gheidary, S.; Akhzari, D.; Pessarakli, M. Effects of salinity, drought, and priming treatments on seed germination and growth parameters of Lathyrus sativus L. J. Plant Nutr. 2017, 40, 1507–1514.
- Asadi, S.; Lebaschy, M.H.; Khourgami, A.; Rad, A.H.S. Effect of drought stress on the morphology of three Salvia sclarea populations. Ann. Biol. Res. 2012, 3, 4503–4507.
- Salazar, C.; Hernández, C.; Pino, M.T. Plant water stress: Associations between ethylene and abscisic acid response. Chil. J. Agric. Res. 2015, 75, 71–79.
- Maleki, M.; Ebrahimzade, H.; Gholami, M.; Niknam, V. The effect of drought stress and exogenous abscisic acid on growth, protein content and antioxidative enzyme activity in saffron (Crocus sativus L.). Afr. J. Biotechnol. 2011, 10, 9068–9075.
- Smith, S.; De Smet, I. Root system architecture: insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. B: 2012; 367, 1441–1452.
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.; Akram, N.A.; Abideen, Z. Morpho-anatomical adaptations of two Tagetes Erecta L. cultivars with contrasting response to drought stress. Pak. J. Bot. 2020, 52, 801–810.
- Akhtar, I.; Nazir, N. Effect of waterlogging and drought stress in plants. Int. J. Water Res. Environ. Eng. 2013, 2, 34–40.
- Bhargavi, B.; Kalpana, K.; Reddy, J.K. Influence of Water Stress on Morphological and Physiological Changes in Andrographis paniculata. Int. J. Pure Appl. Biosci. 2017, 5, 1550–1556.
- Najla, S.; Sanoubar, R.; Murshed, R. Morphological and biochemical changes in two parsley varieties upon water stress. Physiol. Mol. Biol. Plants 2012, 18, 133–139.
- Srivastava, S.; Srivastava, M. Morphological changes and antioxidant activity of Stevia rebaudiana under water stress. Am. J. Plant Sci. 2014, 5, 3417.
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean [Vigna radiata (L.) Wilczek] varieties at different developmental stages under drought stress. Turk. J. Biol. 2019, 43, 58–69.
- Deka, D.; Singh, A.K.; Singh, A.K. Effect of Drought Stress on Crop Plants with Special Reference to Drought Avoidance and Tolerance Mechanisms: A Review. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2703–2721.
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 610721, doi.org/10.1155/2013/610721
- Zare, M.; Azizi, M.H.; Bazrafshan, F. Effect of drought stress on some agronomic traits in ten barley (Hordeum vulgare L.) cultivars. Tech. J. Eng. Appl. Sci. 2011, 1, 57–62.
- Bhargava, S.; Sawant, K. Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32.
- Singh, J.; Thakur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and abiotic stress tolerance in plants, Springer: Gateway East Singapore, Singapore, 2018; pp. 27-46.
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461.
- Chaves, M.M.; Flexas, J. Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560.
- Deepak, S.B.; Thakur, A.; Singh, S.; Bakshi, M.; Bansal, S. Changes in crop physiology under drought stress: A review. J. Pharmacogn. Phytochem. 2019, 8, 1251–1253.
- Sicher, R.C.; Barnaby, J.Y. Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol. Planta. 2012, 144, 238–253.
- Rahmati, M.; Mirás-Avalos, J.M.; Valsesia, P.; Lescourret, F.; Génard, M.; Davarynejad, G.H.; Bannayan, M.; Azizi, M.; Vercambre, G. Disentangling the effects of water stress on carbon acquisition, vegetative growth, and fruit quality of peach trees by means of the QualiTree model. Front. Plant Sci. 2018, 9, 3.
- Zsófi, Z.; Tóth, E.; Rusjan, D.; Bálo, B. Terroir aspects of grape quality in a cool climate wine region: Relationship between water deficit, vegetative growth and berry sugar concentration. Scient. Hortic. 2011, 127, 494–499.
- Ge, T.; Sui, F.; Bai, L.; Tong, C.; Sun, N. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol. Planta. 2012, 34, 1043–1053.
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res, 6, 1539-1547. J. Med. Plants Res. 2012, 6, 1539–1547.
- Anjum, S.A.; Xie, X.-y.; Wang, L.-c.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032.
- Chowdhury, J.; Karim, M.; Khaliq, Q.; Ahmed, A. Effect of drought stress on bio-chemical change and cell membrane stability of soybean genotypes. Bangladesh J. Agr. Res. 2017, 42, 475–485.
- Xiang, D.-B.; Peng, L.-X.; Zhao, J.-L.; Zou, L.; Zhao, G.; Song, C. Effect of drought stress on yield, chlorophyll contents and photosynthesis in tartary buckwheat (Fagopyrum tataricum). J. Food Agric. Environ. 2013, 11, 1358–1363.
- Karimpour, M. Effect of Drought Stress on RWC and Chlorophyll Content on Wheat (Triticum Durum L.) Genotypes. World. Ess. J. 2019, 7, 52-56.
- Abid, G.; M’hamdi, M.; Mingeot, D.; Aouida, M.; Aroua, I.; Muhovski, Y.; Sassi, K.; Souissi, F.; Mannai, K.; Jebara, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 536–552.
- Gurumurthy, S.; Sarkar, B.; Vanaja, M.; Lakshmi, J.; Yadav, S.; Maheswari, M. Morpho-physiological and biochemical changes in black gram (Vigna mungo L. Hepper) genotypes under drought stress at flowering stage. Acta Physiol. Plant. 2019, 41, 42.
- Cuellar‐Ortiz, S.M.; De La Paz Arrieta‐Montiel, M.; Acosta‐Gallegos, J.; Covarrubias, A.A. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008, 31, 1399–1409.
- Abdallah, M.B.; Trupiano, D.; Polzella, A.; De Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Youssef, N.B.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95.
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232.
