Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Neuroinflammation is defined as one of the key contributors involved in several CNS-related disorders including neurodegenerative diseases. According to experimental evidence, the inflammatory process in the neuron has been shown to cause cell death and neurodegeneration in Parkinson’s (PD), Alzheimer’s (AD), and other neurodegenerative diseases.
- clobenpropit
- neurodegenerative diseases
1. Introduction
Neuroinflammation and mitochondrial dysfunction also have a key role in AD and other neurodegenerative-related disorders [3,4]. In the brain, neuroinflammation is mediated by several factors, including cytokines, prostaglandin E2, oxidative stress, and reactive nitrogen species [5]. Furthermore, inflammatory mediators, particularly the cytokine TNF-α, can change cellular mitochondrial metabolism by inhibiting mitochondrial oxidative phosphorylation and related ATP synthesis while also initiating mitochondrial reactive oxygen species formation. On the other hand, when damaged mitochondria are not appropriately eliminated by mitophagy, their contents can leak into the cytosol and extracellular environment, aggravating the inflammatory responses in brain tissue [6].
Central or peripheral administration of an endotoxin, lipopolysaccharide (LPS), results in neuroinflammation by inducing the releases of cytokines including TNF-α, IL-1β, and IL-6 through activation of microglia [7]. Furthermore, the elevation of these cytokines induces the proliferation of APP expression and amyloidogenesis in AD [8]. A previous study showed that LPS stimulated the expression of neuroinflammatory markers due to differences in oxidative stress, oxidative phosphorylation, and mitochondrial activities. The mitochondrial complex activity and mitochondrial membrane potential could be influenced by oxidative stress [1]. Additionally, a bilateral intracerebroventricular injection of LPS established mitochondrial electron transfer chain dysfunction by decreasing the MRCC (I, IV, and V) activities [5].
The list of antagonists on HH3R has gained substantial attention in recent years as a potential treatment for CNS-related disorders including AD. These receptors are found in pre-synaptic neurons as auto-receptors as well as heteroreceptors and control the release of neurotransmitters including histamine [3]. Antagonizing the HH3R facilitates the pre-synaptic releases of several neurotransmitters including histamine, acetylcholine, norepinephrine, and dopamine, which have a major role in various CNS functions [9]. CLO is a potent HH3R antagonist and has been reported to augment the pre-synaptic release of neurotransmitters including histamine, acetylcholine, dopamine, and noradrenaline [10,11,12,13]. Recently, in a rat model of AD, administration of CLO (1 mg/kg, i.p.) was found to have a neuroprotective effect against Aβ-peptide infusion-induced neuronal toxicity [14]. Furthermore, in rats, a bilateral intrahippocampal injection of CLO improves spatial memory deficits caused by MK-801 via modulating the levels of different neurotransmitters [15].
2. CLO Improved Memory Functions in LPS-Treated Mice Using the RAM
Memory functions of LPS-challenged mice pre-treated with CLO were examined in terms of selected three behavioral parameters such as TTB, WME, and RME in the RAM test. Figure 1A shows the effect of CLO on four days of TTB assessment in LPS-induced memory impairment. Analyzing by one-way ANOVA and comparing among the groups, statistical differences were found in TTB (F(3,20) = 17.87, p < 0.001 for day 1; F(3,20) = 9.030, p < 0.001 for day 2; F(3,20) = 11.74, p < 0.001 for day 3; and F(3,20) = 25.95, p < 0.001 for day 4). Furthermore, multiple post hoc analyses indicated that LPS-treatment extensively increased (p < 0.001) the TTB for four days as compared with the control, representing the memory decline by LPS injections. However, CLO at doses of 1 mg/kg (p < 0.001, day 1 and day 4; p < 0.01, day 2 and day 3) and 3 mg/kg (p < 0.001, day 1, day 3, and day 4; p < 0.01, day 2) significantly reduced the LPS-induced TTB increase.
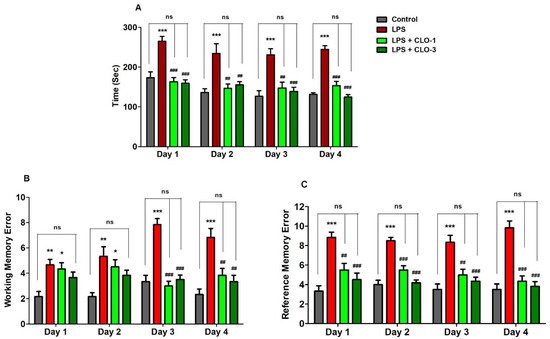
Figure 1. Effect of the clobenpropit (CLO) on the (A) time taken to consume all five baits (TTB), (B) working memory error (WME), and (C) reference memory error (RME) on day 1 to day 4 of memory assessment in lipopolysaccharide (LPS)-induced mice using radial arm maze. LPS-CLO-1 and LPS-CLO-3 refer to administration of clobenpropit (1 or 3 mg/kg, p.o., respectively) and lipopolysaccharides (250 μg/kg, i.p.). TTB, WME and REM were increased by LPS-induced neuroinflammation. However, CLO treatment significantly reduced the LPS-induced TTB, WME and REM increments. The results are expressed as mean ± SEM (n = 6). One-way ANOVA (TTB; F(3,20) = 17.87, p < 0.001 for day 1; F(3,20) = 9.030, p < 0.001 for day 2; F(3,20) = 11.74, p < 0.001 for day 3; and F(3,20) = 25.95, p < 0.001 for day 4), (WME; F(3,20) = 6.460, p < 0.01 for day 1; F(3,20) = 6.272, p < 0.01 for day 2; F(3,20) = 28.97, p < 0.001 for day 3; and F(3,20) = 12.39, p < 0.001 for day 4) (REM; F(3,20) = 14.88, p < 0.001 for day 1; F(3,20) = 29.21, p < 0.001 for day 2; F(3,20) = 13.41, p < 0.001 for day 3; and F(3,20) = 26.52, p < 0.001 for day 4) was followed by Tukey–Kramer multiple comparisons tests.* p < 0.05, ** p < 0.01, and *** p < 0.001 as compared to the control group; ns, not significant as compared to the control group; ## p < 0.01 and ### p < 0.001 as compared to the LPS-treated group.
As shown in Figure 1B, when comparing among groups, significant variations were noted in the number of WME from day 1 to day 4 (F(3,20) = 6.460, p < 0.01; F(3,20) = 6.272, p < 0.01; F(3,20) = 28.97, p < 0.001; F(3,20) = 12.39, p < 0.001, respectively). Administration of LPS by peripheral injections impaired the working memory by increasing the WME (p < 0.01, day 1 and day 2; p < 0.001, day 3 and day 4) throughout the experiment. Pre-administration of CLO, however, considerably reduced the number of WME only on day 3 and day 4 (p < 0.001 and p < 0.01 at 1 and 3 mg/kg, p.o., respectively).
In addition, the reference memory was also altered with different groups of treatments (F(3,20) = 14.88, p < 0.001 for day 1; F(3,20) = 29.21, p < 0.001 for day 2; F(3,20) = 13.41, p < 0.001 for day 3; and F(3,20) = 26.52, p < 0.001 for day 4) by using one-way ANOVA analysis (Figure 1C). When matched to control animals, it was indicated that the LPS-treated mice group showed higher numbers of RMEs (p < 0.001) after four days of the experiments. Nevertheless, additional treatment of CLO with the LPS treatment extensively reduced the number of RMEs at the oral dose levels of 1 mg/kg (p < 0.01, day 1 and day 3; p < 0.001, day 2 and day 4) and 3 mg/kg (p < 0.001, from day 1 to day 4).
3. CLO Reduced Pro-Inflammatory Cytokine Levels in LPS-Treated Mice
Figure 2A demonstrates the effects of CLO on two selective pro-inflammatory cytokine markers, TNF-α and IL-6. Comparing all the groups, considerable differences were found in TNF-α levels (F(3,20) = 16.68, p < 0.001) by analyzing with one-way ANOVA. Compared to the control, the LPS-challenged group elicited significantly higher (p < 0.001) TNF-α levels in the brain. CLO treatment (3 mg/kg, p.o.) considerably reduced (p < 0.05) the production of TNF-α in mice brains, as compared to the LPS-challenged group. There were no obvious changes in TNF-α levels with administration of CLO (1 mg/kg, p.o.) in LPS-challenged mice.
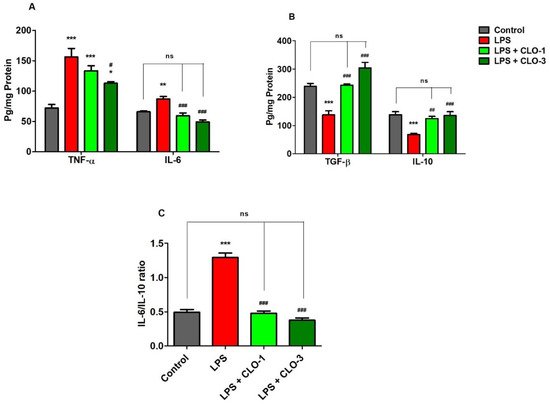
Figure 2. Effect of clobenpropit (CLO) on (A) pro-inflammatory cytokines (TNF-α and IL-6), (B) anti-inflammatory cytokines (TGF-β and IL-10), and (C) IL-6/IL-10 ratio in lipopolysaccharide (LPS)-induced mice brains. LPS-CLO-1 and LPS-CLO-3 refer to the administration of clobenpropit (1 or 3 mg/kg, p.o, respectively) and lipopolysaccharides (250 μg/kg, i.p.). Administration of LPS resulted in the elevation of pro-inflammatory cytokines and reduction of anti-inflammatory cytokine activities. Interestingly, the CLO treatment attenuated the inflammatory activity of LPS. The ratio of IL-6/IL-10 also lowered with CLO treatment in LPS-challenged mice. The results are expressed as mean ± SEM (n = 6). One-way ANOVA (F(3,20) = 16.68, p < 0.001 for TNF-α and F(3,20) = 20.96, p < 0.001 for IL-6) (F(3,20) = 26.33, p < 0.001 for TGF-β and F(3,20) = 11.18, p < 0.001 for IL-10) (F(3,20) = 88.18, p < 0.001 for IL-6/IL-10 ratio) was followed by Tukey–Kramer multiple comparisons tests. * p < 0.05, ** p < 0.01 and *** p < 0.001 as compared to the control group; ns, not significant as compared to the control group; # p < 0.05, ## p < 0.01 and ### p < 0.001 as compared to the LPS-treated group.
The effect of CLO on brain IL-6 production in LPS-treated mice is shown in Figure 2A. Referring to the results, when comparing among groups, differences in IL-6 levels (F(3,20) = 20.96, p < 0.001) were found in brain homogenates. Multiple post hoc comparisons showed that LPS administration significantly elevated (p < 0.01) IL-6 levels in brain tissues of the control animals. However, treatment with CLO (1 and 3 mg/kg, p.o.) suggestively (p < 0.001) reversed it by reducing the brain IL-6 levels when compared with the LPS-treated group, and both doses almost equalized the IL-6 levels of the control.
4. CLO Improved Anti-Inflammatory Cytokine Levels in LPS-Treated Mice
Figure 2B shows the effects of CLO on anti-inflammatory cytokine markers TGF-β1 and IL-10 in brain homogenates of LPS-treated mice. There were extensive differences noted when comparing among the groups of TGF-β1 levels (F(3,20) = 26.33, p < 0.001). The levels of TGF-β1 in LPS-injected mice were significantly (p < 0.001) lower compared with control mice. The results indicated that LPS-treatment lowered the anti-inflammatory activity in the mouse brain. Pretreatment with 1 and 3 mg/kg of CLO, however, significantly improved TGF-β1 levels in LPS-challenged mice (p < 0.01 and p < 0.001, respectively). There were no notable changes between the control and both CLO-treated groups.
For IL-10 levels, there were significant variations recorded (F(3,20) = 11.18, p < 0.001) among the groups (Figure 2B). When compared with the control group, it was shown that LPS treatment resulted in a significantly lower level of IL-10 (p < 0.001) in mice brains. Nevertheless, CLO (1 and 3 mg/kg, p.o.) pre-treatment substantially elucidated (p < 0.01 and p < 0.001) the brain IL-10 levels in LPS-challenged animals.
Furthermore, the effects of CLO on the IL-6/IL-10 ratio in LPS-treated mice are shown in Figure 2C. The IL-6/IL-10 ratio was significantly (p < 0.001) higher in LPS-treated mice than in the control. This indicated the higher activity of pro-inflammatory cytokines with LPS treatment. However, treatment with 1 and 3 mg/kg of CLO significantly (p < 0.001) lowered the IL-6/IL-10 ratio in LPS-challenged mice.
5. CLO Reduced Cyclooxygenase-2 (COX-2) Activities in LPS-Treated Mice
Figure 3 refers to the effects of CLO on brain COX-2 levels of LPS-treated mice. While comparing among groups, extensive differences were shown for COX-2 levels (F(3,20) = 33.67, p < 0.001) using one-way ANOVA analysis. Furthermore, the comparison between selected groups showed a significant raise (p < 0.001) in COX-2 enzyme levels of LPS-challenged mice brains compared to the control group. However, oral treatment with CLO considerably reduced (p < 0.001) COX-2 activity in LPS-challenged mice. In both CLO treatment groups, the COX-2 levels were similar to the control animals.
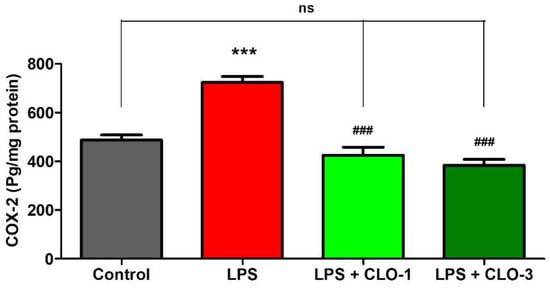
Figure 3. Effect of clobenpropit (CLO) on cyclooxygenase-2 (COX-2) levels in lipopolysaccharide (LPS)-induced mice brains. LPS-CLO-1 and LPS-CLO-3 refer to administration of clobenpropit (1 or 3 mg/kg, p.o., respectively) and lipopolysaccharides (250 μg/kg, i.p.). CLO treatment reduced the activity of COX-2 induced by LPS. The results are expressed as mean ± SEM (n = 6). One-way ANOVA (F(3,20) = 33.67, p < 0.001) was followed by Tukey–Kramer multiple comparisons tests. *** p < 0.001 as compared to the control group; ns, not significant as compared to the control group; ### p < 0.001 as compared to the LPS-treated group.
6. CLO Improved Mitochondrial Respiratory Chain Complexes (MRCC) Activities in LPS-Treated Mice
Figure 4 shows the effect of LPS and CLO treatment on various MRCC activities. Regarding MRCC-I, one-way ANOVA analysis showed that there was considerable variation (F(3,20) = 10.76, p < 0.001) among the groups (Figure 4A). Post hoc analysis revealed that i.p. injection of LPS caused a significant decrease (p < 0.01) in MRCC-I activity in mice brains. Nevertheless, treatment with CLO at 3 mg/kg (p < 0.001) attenuated the LPS-induced declined MRCC-I activity in the brain. There were no changes in brain MRCC-I activity when compared with LPS-treatment.
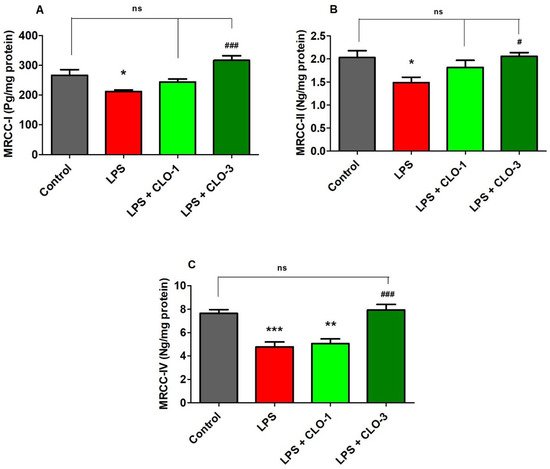
Figure 4. Effect of clobenpropit (CLO) on mitochondrial respiratory chain complex (MRCC) (A) MRCC-I, (B) MRCC-II, and (C) MRCC-IV activities in lipopolysaccharides (LPS)-treated mice brains. LPS-CLO-1 and LPS-CLO-3 refer to the administration of clobenpropit (1 or 3 mg/kg, p.o., respectively) and lipopolysaccharides (250 μg/kg, i.p.). MRCC levels were decreased with administration of LPS injection and the levels were increased with oral administration of CLO. The results are expressed as mean ± SEM (n = 6). One-way ANOVA (F(3,20) = 10.76, p < 0.001 for MRCC-I; F(3,20) = 4.270, p < 0.05 for MRCC-II; F(3,20) = 16.85, p < 0.001 for MRCC-IV) was followed by Tukey–Kramer multiple comparisons tests. * p < 0.05, ** p < 0.01 and *** p < 0.001 as compared to the control group; ns, not significant as compared to the control group; # p < 0.05 and ### p <0.001 as compared to the LPS-treated group.
Results from MRCC-II activity (Figure 4B), when compared among the groups, show that there were significant changes in MRCC-II activity (F(3,20) = 4.270, p < 0.05) among the groups in mice brains. Furthermore, administration of LPS caused a considerable decrease in MRCC-II activity as related to control animals. Similarly, the higher dose of CLO (3 mg/kg, p.o.) significantly improved the brain MRCC-II activity (p < 0.05) that was decreased by injections of LPS. At a low dose of CLO (1 mg/kg, p.o.), no alteration in the level of brain MRCC-II activity resulted as compared to LPS-treated mice.
It was found (Figure 5C) that there were notable variations among the groups for brain MRCC-IV activity (F(3,20) = 16.85, p < 0.001) in mice. When compared to the control group, the LPS-treatment declined the MRCC-IV activity (p < 0.001) in brain tissues. Interestingly, treatment with both doses (1 and 3 mg/kg, p.o.) of CLO reversed the MRCC-IV levels (p < 0.01 and p < 0.001, respectively) in a dose-dependent manner in mice brains.
Overall, it demonstrated that using a mouse model, the HH3R antagonist clobenpropit could act as a promising neuroprotective target against cognitive impairment, neuronal inflammation, and mitochondrial damage in the brain. Clobenpropit showed improvement in spatial learning and memory in the RAM test. It also showed anti-inflammatory potential by attenuating LPS-induced elevation of COX-2 enzymes as well as pro-inflammatory cytokine levels (TNF-α, and IL-6) levels and also increased anti-inflammatory cytokine levels (TGF-β1 and IL-10) in the mouse brain. Furthermore, pre-treatment with clobenpropit (3 mg/kg, p.o.) improved the MRCC (I, II, and IV) functions in LPS-challenged mice. The achieved results underline that clobenpropit could be a promising drug in the prevention of neuroinflammatory insults in various neurodegenerative diseases.
This entry is adapted from the peer-reviewed paper 10.3390/brainsci11121617
This entry is offline, you can click here to edit this entry!
