Metal-based nanomaterials could be widely used in biomedical fields, as metal ions are essential in living organisms. Since the particle size of the virus particles ranges from tens to hundreds of nanometers, the surface activity of the metal material is enhanced after the metal is nanosized. It can be used in the inhibition of virus infection.
- metal-based nanomaterials
- characteristics
- antiviral therapy
- mechanism
- application
1. Introduction
Virus infection has always been a threat to human and animal health. Typical viruses include hepatitis B virus [1], influenza virus [2], human immunodeficiency virus (HIV) [3], and coronavirus [4], etc., which can cause severe disease. Therefore, antiviral drug development is a major research direction for scientists. At present, the main treatment methods for viral infections include developing vaccines and screening antiviral drugs. However, the cycle for virus vaccine development and drug screening is currently too long. Additionally, the emergence of antiviral drug resistance has brought considerable challenges to successfully suppressing viral infections, so the development of new antiviral drugs is particularly important.
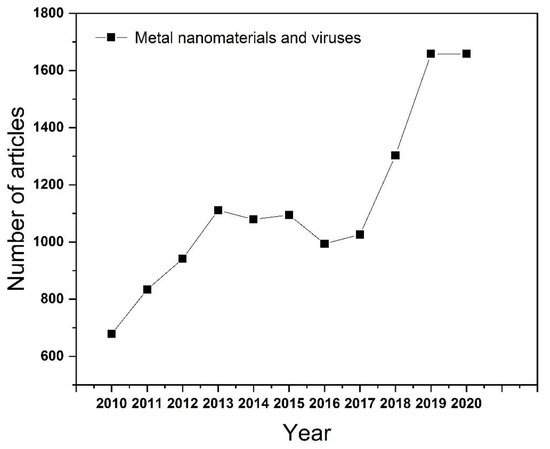
As one of the emerging fields in recent years, nanomaterials can be simply divided into one-dimensional, two-dimensional, and three-dimensional nanostructures according to the morphology of the material. There are many methods to synthesize nanomaterials, such as mechanical grinding synthesis [5], chemical vapor synthesis [6], chemical liquid reaction [7], physical vapor deposition [8], and phytosynthesis [9][10]. Among them, the phytosynthesis method is the most remarkable method of preparing nanoparticles now. It has the advantages of environmental protection and low energy consumption, but it also has the disadvantages of limited reaction yield and poor particle uniformity. Since then, further research has made substantial progress on the preparation methods of nanomaterials by adjusting the ratio of raw materials and exploring suitable reaction conditions. Nanomaterials have the advantages of small size, high specific surface area, adjustable particle size, and easier surface functionalization, which make them widely used in sensing [11], catalysis [12], energy storage [13], and the medical treatment field [14]. Metal-based nanomaterials could be widely used in biomedical fields, as metal ions are essential in living organisms. Since the particle size of the virus particles ranges from tens to hundreds of nanometers, the surface activity of the metal material is enhanced after the metal is nanosized. It can be used in the inhibition of virus infection. In addition, according to the statistics on Web of Science, the number of articles published on the application of metal nanomaterials in the field of viruses has been increasing year-on-year ( Figure 1 ). More importantly, some nanomaterials such as Ag [15], Au [16], ZnO [17], etc., which have an inhibitive effect on bacteria and viruses, highlight their potential for antiviral applications. Notably, the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019 poses a huge threat to human health. As such, progress on virus pathogenesis new antiviral drug development is a research priority [18].
2. Inhibition of Virus Infections
Virus infection of cells can be divided into three stages. The first is the early adsorption of the virus onto cell membrane, where the virus binds to cell surface receptors through its surface protein. The second is the process of uncoating, replication, and translation after the virus enters the cell. Finally, the virus repackages to release new progeny viruses. In these processes, if metal nanomaterials can inhibit the virus in the early stage and prevent the virus from invading cells, they can be used in medical protective equipment, which has great potential for preventing the virus spread ( Figure 2 ).
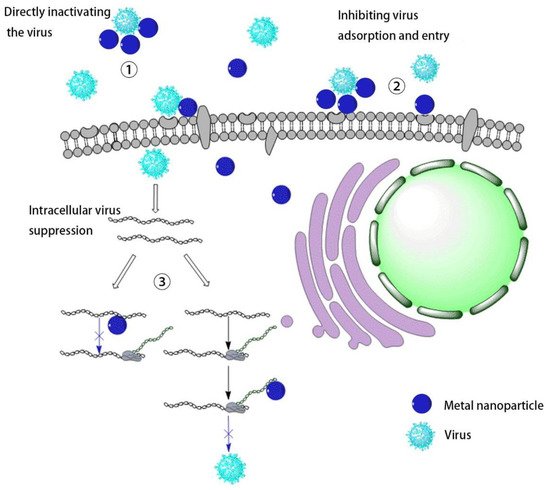
Compared with some metal-based nanomaterials that use light to produce ROS to inactivate viruses, the mutual combination of metal ions and protein molecules can often change the protein conformation, causing irreversible damage to the effect of inhibiting virus infection. SungJun Park synthesized a magnetic hybrid colloid loaded with Ag nanoparticles of different sizes. Using the interaction between Ag and biological macromolecules, this nanomaterial’s inhibitory effect on bacteriophages, norovirus, and adenovirus was explored. The results show that the virus binds to the sulfhydryl-containing protein on the surface of the virus through Ag nanoparticles, thereby destroying the virus envelope to inhibit the virus. Similarly, the use of the affinity of metal ions with proteins can bind to the outer surface proteins of virus particles and destroy the virus structure to inhibit the virus [19]. F. Pfaff tested its inhibitory effect on modified vaccinia virus Ankara (MVA), human adenovirus serotype 5 (HAdV-5), poliovirus type 1 (PV-1), and murine norovirus (MNV) by co-cultivating WC material and virus. The author shows that WC tends to reunite, which can encapsulate virus particles, thereby destroying the nucleic acid of the virus and inactivating the virus [20].
The process of a virus invading cells involves specific binding, which provides a good starting point for preventing viruses from infecting cells. The primary inhibition method is to competitively bind to the virus by simulating cell surface virus receptors and inhibiting the virus from invading cells. However, this method can stop the virus from spreading further and does not inactivate the virus. There are certain drawbacks to the application. It is worth noting that these nanomaterials can be adsorbed on medical protective fabrics and concrete surfaces, thereby reducing the spread and infection of viruses.
After the virus enters the cell, it uses the intracellular machinery to carry out protein replication and translation. Since the virus is uncoated to expose the genetic material DNA/RNA, this process can provide the possibility for external drugs to destroy the viral nucleic acid or interfere with the process of translation, replication, and release.
3. Loading Drug Synergy
The poor water solubility of antiviral drugs makes their bioavailability low, and higher doses are often required to achieve the desired therapeutic effect. However, higher doses will produce certain toxicity to organisms, so the emergence of drug carriers can improve the drugs’ bioavailability and reduce the damage to organisms. When selecting metal nanoparticles as drug carriers, the toxicity of metal ions to organisms and the loading efficiency of materials should be considered. Using metal ions or inert ions, with higher content in human cells, can reduce the toxicity of materials and increase the materials’ natural metabolism.
Quantum dots (QD) are a type of low-dimensional semiconductor material and often have smaller sizes. Therefore, applying QD materials to the field of biological therapy can often improve the therapeutic effect. Ranjeet Dungdung’s group used ZnS quantum dots as a drug carrier, loaded with mycophenolic acid (MPA), an immunosuppressant against dengue fever virus. It was found that cells have a higher internalization rate of ZnS-coupled MPA, and its inhibitory effect on dengue virus was significantly improved and the selectivity index was increased by two orders of magnitude [21]. The study shows that quantum dots can significantly increase the uptake rate and therapeutic effect of drugs, indicating that QD as drug carriers have great application prospects.
Vaccines can be divided into live-attenuated vaccines, inactivated vaccines, recombinant vaccines, and so on. Among them, live vaccines (adenovirus vaccines [22], measles vaccines [23], and polio vaccines [24]) can stimulate the body’s comprehensive systemic immunity and long-lasting immune response with the disadvantages of antigen interference and enhanced virulence. Inactivated vaccine (influenza split vaccine [25], rabies vaccine [26], and hepatitis A vaccine [27]) are viruses that have been inactivated by chemical or physical methods, and still maintain the immunogenicity of their immune antigens. Killed vaccines have high safety and good stability. However, adjuvants are often needed to enhance the immune effect. Gene vaccines (hepatitis B vaccine [28], HIV vaccine [29]) are not infectious, convenient for mass production, and safe. They also need adjuvants to enhance the immune effect during usage. Therefore, adding vaccine adjuvants to non-specifically increase the body’s immune response in traditional vaccine production is often a good preparation plan. These adjuvants often increase the immune response ability in organisms through their own physical and chemical properties or by changing antigens’ physical properties.
4. Conclusions
The spread of viruses such as SARS-CoV and SARS-CoV-2 as well as influenza viruses poses a huge threat to human health. It is generally known that viruses are prone to mutate due to external influences to produce different virus subtypes, limiting the use of traditional antiviral drugs and vaccines. Therefore, novel drug discovery and vaccine development for the treatment of viral infectious diseases is very important.
Metal-based nanomaterials have the advantages of high specific surface area and small particle size, making them widely used in the biological field. In terms of antiviral activity, some gold-based nanomaterials such as Au, Ag, CuI, TiO 2, etc. , have virus-inactivating ability and can damage their surface proteins or their genetic material by combining with viruses. In comparison, nanoparticles such as ZnO and Fe 3O 4 can prevent the virus from further infecting cells by interfering with the replication, translation, and release of the virus. Some other metal nanoparticles have a small particle size and good biocompatibility, are easily taken up by cells, and can be used as a suitable carrier for antiviral drugs. Moreover, utilizing the binding of metal ions and biological macromolecules, metal nanoparticles can be used as vaccine adjuvants or adjuvant carriers to promote the occurrence of immune response of the body. However, there are still some problems to be solved in the antiviral application of metal-based nanomaterials. First of all, the toxicity of metal ions in organisms is still a major obstacle to their application. The biological toxicity of metal ions has always been a major obstacle to the application of metal-related materials in biological treatments. While ensuring their therapeutic effect, reducing the in vivo toxicity of metal ions is one of the most important directions for researchers to explore. At this stage, there are mainly two methods; first, by reducing the concentration of metal materials used, and second, by optimizing the metabolism of metal ions. However, the effect is not yet satisfactory. Next, the antiviral mechanism of some metal ions has not yet been explored clearly. Finally, there are many metal nanomaterials, and the current research is limited to a few metal ions. The antiviral properties of other metals still need to be further studied. Based on their excellent properties, the application of metal-based nanomaterials in the antiviral field is a promising research field for expansion.
This entry is adapted from the peer-reviewed paper 10.3390/nano11082129
References
- Tan, M.; Bhadoria, A.S.; Cui, F. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 106–119.
- Beerens, N.; Germeraad, E.A.; Venema, S.; Verheij, E.; Pritz-Verschuren, S.B.E.; Gonzales, J.L. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg. Microbes Infect. 2021, 10, 97–108.
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.S.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505.
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Shen, L.; Bao, N.; Yanagisawa, K.; Domen, K.; Gupta, A.; Grimes, C.A. Direct synthesis of ZnO nanoparticles by a solution-free mechanochemical reaction. Nanotechnology 2006, 17, 5117–5123.
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428.
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881.
- Pascu, A.; Stanciu, E.M.; Croitoru, C.; Roata, I.C.; Tierean, M.H. Carbon Nanoparticle-Supported Pd Obtained by Solar Physical Vapor Deposition. Adv. Mater. Sci. Eng. 2018, 2018, 1–7.
- Rai, M.; Yadav, A.; Gade, A. CRC 675—Current Trends in Phytosynthesis of Metal Nanoparticles. Crit. Rev. Biotechnol. 2008, 28, 277–284.
- Mohamed, H.; Afridi, S.; Khalil, A.T.; Zohra, T.; Alam, M.M.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Phytosynthesis of BiVO4 nanorods using Hyphaene thebaica for diverse biomedical applications. AMB Express 2019, 9, 1–14.
- Howes, P.D.; Chandrawati, R.; Stevens, M.M.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390.
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251.
- Tale, B.; Nemade, K.R.; Tekade, P.V. Graphene based nano-composites for efficient energy conversion and storage in Solar cells and Supercapacitors: A Review. Polym. Plast. Technol. Mater. 2021, 60, 784–797.
- Liang, G.F.; Wang, H.J.; Shi, H.; Wang, H.T.; Zhu, M.X.; Jing, A.H.; Li, J.H.; Li, G.D. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 154.
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R.P. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732.
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 413–421.
- Mishra, Y.K.; Adelung, R.; Röhl, C.; Shukla, D.; Spors, F.; Tiwari, V. Virostatic potential of micro–nano filopodia-like ZnO structures against herpes simplex virus-1. Antivir. Res. 2011, 92, 305–312.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral Properties of Silver Nanoparticles on a Magnetic Hybrid Colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350.
- Pfaff, F.; Glück, B.; Hoyer, T.; Rohländer, D.; Sauerbrei, A.; Zell, R. Tungsten carbide nanoparticles show a broad spectrum virucidal activity against enveloped and nonenveloped model viruses using a guideline-standardized in vitro test. Lett. Appl. Microbiol. 2019, 69, 302–309.
- Dungdung, R.; Bayal, M.; Valliyott, L.; Unniyampurath, U.; Nair, S.S.; Pilankatta, R. A slow, efficient and safe nanoplatform of tailored ZnS QD-mycophenolic acid conjugates for in vitro drug delivery against dengue virus 2 genome replication. Nanoscale Adv. 2020, 2, 5777–5789.
- Kuschner, R.A.; Russell, K.L.; Abuja, M.; Bauer, K.M.; Faix, D.J.; Hait, H.; Henrick, J.; Jacobs, M.; Liss, A.; Lynch, J.A.; et al. A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine 2013, 31, 2963–2971.
- Simon, J.K.; Pasetti, M.F.; Viret, J.-F.; Munoz, A.; Lagos, R.; Levine, M.M.; Campbell, J.D. A Clinical Study to Assess the Safety and Immunogenicity of Attenuated Measles Vaccine Administered Intranasally to Healthy Adults. Hum. Vaccin. 2014, 3, 54–58.
- Eswaran, S.P.; Praharaj, A.K.; Chander, Y.; Nagendra, A. Potency Titration of Oral Polio Vaccine by Estimation of Live Virus Content Using Tissue Culture Technique. Med. J. Armed Forces India 2003, 59, 105–107.
- Delore, V.; Salamand, C.; Marsh, G.; Arnoux, S.; Pepin, S.; Saliou, P. Long-term clinical trial safety experience with the inactivated split influenza vaccine, Vaxigrip®. Vaccine 2006, 24, 1586–1592.
- Finke, S.; Karger, A.; Freuling, C.; Muller, T. Assessment of inactivated human rabies vaccines: Biochemical characterization and genetic identification of virus strains. Vaccine 2012, 30, 3603–3609.
- Zhang, Z.; Zhu, X.; Hu, Y.; Liang, M.; Sun, J.; Song, Y.F.; Yang, Q.; Ji, H.Q.; Zeng, G.; Song, L.F.; et al. Five-year antibody persistence in children after one dose of inactivated or live attenuated hepatitis A vaccine. Hum. Vaccin. Immunother. 2017, 13, 1–6.
- Hernandez-Bernal, F.; Aguilar-Betancourt, A.; Aljovin, V.; Arias, G.; Valenzuela, C.; De Alejo, K.P.; Hernandez, K.; Oquendo, O.; Figueredo, N.; Figueroa, N.; et al. Comparison of four recombinant hepatitis B vaccines applied on an accelerated schedule in healthy adults. Hum. Vaccin. 2011, 7, 1026–1036.
- Ellenberger, D.; Li, B.; Smith, J.; Yi, H.; Folks, T.; Robinson, H.; Butera, S. Optimization of a multi-gene HIV-1 recombinant subtype CRF02_AG DNA vaccine for expression of multiple immunogenic forms. Virology 2004, 319, 118–130.
