Sodium metabisulfite (MBS) was used in this study for selective flotation of chalcopyrite and molybdenite. Microflotation tests of single and mixed minerals were performed to assess the floatability of chalcopyrite and molybdenite. The results of microflotation of single minerals showed that MBS treatment significantly depressed the floatability of chalcopyrite and slightly reduced the floatability of molybdenite. The results of microflotation of mixed minerals demonstrated that the MBS treatment could be used as a selective chalcopyrite depressant in the selective flotation of chalcopyrite and molybdenite. Furthermore, the addition of diesel oil or kerosene could significantly improve the separation efficiency of selective flotation of chalcopyrite and molybdenite using MBS treatment.
1. Introduction
Molybdenum minerals are often associated with copper sulfide minerals [
1]. It is estimated that about 50% of the world’s molybdenum production comes from copper and molybdenum (Cu-Mo) ores as a by-product [
2,
3]. Both copper and molybdenum are important materials in various fields; therefore, the separation of both copper and molybdenum minerals is important. Furthermore, molybdenum minerals play a very important role in making the Cu-Mo processing plant economically viable [
4].
Separation of copper and molybdenum sulfide minerals is often carried out in the selective flotation stage by adding a copper depressant (i.e., sodium hydrosulfide (NaHS), sodium sulfide (Na
2S), sodium thiopropionate (HSCH
2CH
2COONa), sodium thioglycollate (HSCH
2COONa), or Nokes reagent (P
2S
5+NaOH)) [
2,
5,
6,
7,
8,
9,
10]. Other reagents have been developed to replace these toxic and dangerous copper depressants, for instance, by using chitosan [
11], lignosulphonate [
12], dithiouracil [
13], and rhodamine-3-acetic acid [
14] as copper depressants in the selective flotation of Cu-Mo sulfide minerals. In addition, various oxidation treatments using plasma pre-treatment, ozone, electrolysis, hydrogen peroxide, and Fenton-like reagent have been applied as selective chalcopyrite depressants in the previous studies [
15,
16,
17,
18,
19]. However, the effectiveness of these reagents in the Cu-Mo flotation plant has not been reported.
Sulfoxy reagents (i.e., sulfite (SO
32−), bisulfite (HSO
3−), metabisulfite (S
2O
52−), or sulfur dioxide (SO
2)) have been used as depressants for various minerals [
20,
21,
22,
23,
24,
25,
26,
27]. These reagents have been commonly used in flotation plants. For instance, sodium sulfite (Na
2SO
3) and sodium metabisulfite (Na
2S
2O
5) are the most widely used compounds for the depression of pyrite during the flotation of copper complex ores [
2,
21,
28,
29]. However, these studies did not report the depression of copper minerals. The selective depressing effect of Na
2SO
3 on the floatability of chalcopyrite has been investigated by Miki et al. [
25] for selective Cu-Mo flotation and by Suyantara et al. [
30] for separation of chalcopyrite and enargite using flotation. However, Miki et al. [
25] showed that the depression of chalcopyrite in the selective Cu-Mo flotation required a high concentration of Na
2SO
3 (i.e., 0.1 M) at pH 10.8, making this reagent practically and economically unattractive.
On the other hand, Castro et al. [
31] showed that sodium metabisulfite has been used to replace lime as a pyrite depressant in the rougher flotation stage of Cu-Mo-Fe ores in Chile. Therefore, it might be more efficient to use sodium metabisulfite for the selective Cu-Mo flotation stage. Chen et al. [
32] studied the effect of sodium metabisulfite on the floatability of molybdenite in fresh water and seawater. They reported that sodium metabisulfite exhibited a depressing effect on the recovery of molybdenite in fresh water. However, the effect of sodium metabisulfite on the depression of chalcopyrite in the selective Cu-Mo flotation has been not reported.
2. Microflotation of Single Mineral
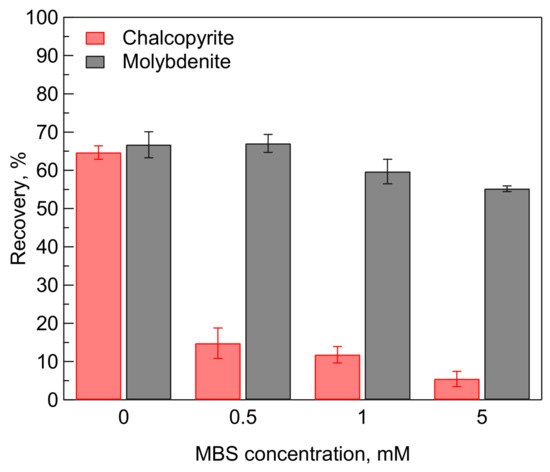
Effects of MBS treatment on the flotation recovery of chalcopyrite and molybdenite in the single mineral system are presented in Figure 1. It should be noted that the microflotation tests were performed in the absence of a collector. The recovery of chalcopyrite significantly decreased from 65% in the absence of MBS to 15% after being treated in 0.5 mM MBS. The recovery of chalcopyrite gradually decreased with increasing concentration of MBS. The recovery of chalcopyrite was 5% after being treated in 5 mM MBS. These microflotation results indicate that the MBS treatment exhibited a strong depressing effect on the floatability of chalcopyrite.
Figure 1. Effects of sodium metabisulfite (MBS) on the recovery of chalcopyrite and molybdenite in the absence of a collector at pH 9.
On the other hand, the addition of 0.5 mM MBS had an insignificant effect on the recovery of molybdenite. However, the addition of a higher concentration of MBS could reduce the recovery of molybdenite. For instance, the recovery of molybdenite slightly decreased from 67% to 55% after being treated in 5 mM MBS. A similar depressing effect of MBS on the floatability of molybdenite in deionized (DI) water has been reported by Chen et al. [
32]. Although these microflotation results indicate that MBS treatment slightly depressed the floatability of molybdenite, the depressing effect was less significant compared to that on the floatability of chalcopyrite. In addition, the microflotation results presented in
Figure 1 show that MBS treatment has potential as a selective copper depressant in the selective flotation of Cu-Mo.
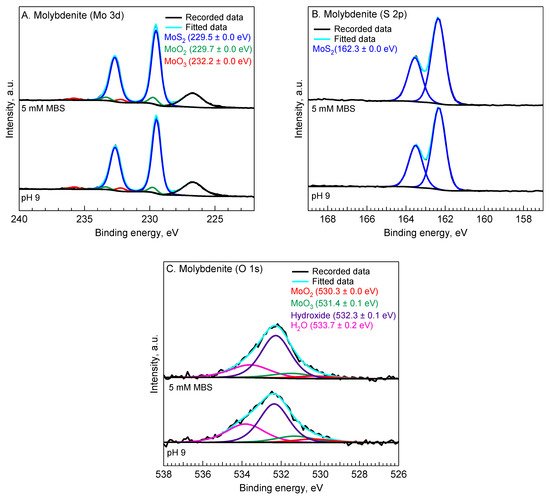
3. XPS Analysis
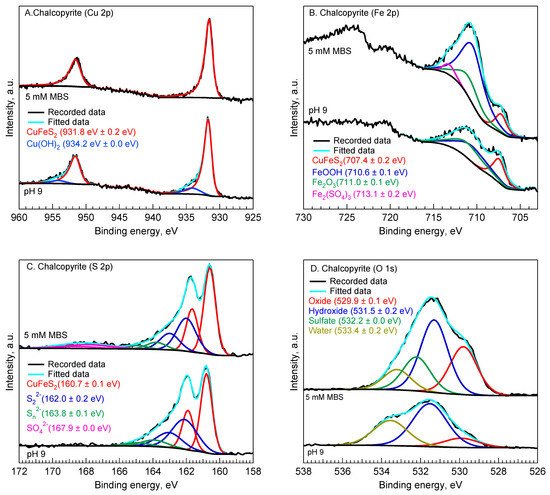
XPS analysis was performed to explain the flotation results presented in Figure 1 by evaluating the oxidation states of chemical species on the surface of chalcopyrite and molybdenite before and after the MBS treatment. Figure 2 shows the X-ray photoelectron spectra of Cu 2p, S 2p, Fe 2p, and O 1s of chalcopyrite.
Figure 2. Cu 2p (A), Fe 2p (B), S 2p (C), O 1s (D) spectra of chalcopyrite without and with 5 mM MBS treatment at pH 9.
Two Gaussian–Lorentzian (GL) functions could best fit the Cu 2p spectrum of chalcopyrite at pH 9 (
Figure 2A). The first GL function shows a peak centered at ca. 931.8 eV, which is in agreement with the Cu(I) of chalcopyrite [
34]. The second GL function shows a peak centered at higher binding energy (i.e., 934.2 eV). This peak is attributed to the Cu(II) of Cu(OH)
2 [
35]. The presence of Cu(II) is confirmed by the formation of a weak satellite peak located at binding energy 940–945 eV. The presence of Cu(II) on the chalcopyrite surface indicates that the chalcopyrite surface was slightly oxidized at pH 9 in the absence of MBS. In addition, the surface oxidation of chalcopyrite at pH 9 is confirmed by the Fe 2p spectrum. The deconvolution of Fe 2p spectrum of chalcopyrite at pH 9 shown in
Figure 2B indicates that in addition to the Fe of chalcopyrite located at 707.4 eV [
36], the chalcopyrite surface was covered by FeOOH and Fe
2O
3, as indicated by the peaks located at 710.6 eV and 711.0 eV, respectively [
37]. The deconvolution of S 2p spectrum of chalcopyrite at pH 9 (
Figure 2C) indicates various peaks located at ca. 160.7 eV, 162.0 eV, and 163.8 eV. These peaks are attributed to chalcopyrite, disulfide (S
22−), and polysulfide (S
n2−), respectively [
36]. The presence of oxide and hydroxide on the chalcopyrite surface is confirmed by the peak located at 529.9 eV [
37] and 531.5 eV [
38] in the O 1s spectrum of chalcopyrite at pH 9 (
Figure 2D). In addition, there is an indication of the presence of adsorbed water on the surface of chalcopyrite, as shown by the peak located at 533.4 eV [
39] in
Figure 2D.
The MBS treatment reduced the Cu(II) to Cu(I) on the chalcopyrite surface, as indicated by the absence of Cu(II) species in the Cu 2p spectrum of chalcopyrite (
Figure 2A). Only the Cu 2p peak of chalcopyrite was observed after the surface was treated in 5 mM MBS. The Fe 2p spectrum in
Figure 2B shows that the MBS treatment significantly improved the peak intensity of FeOOH and formed a new peak located at 713.1 eV. This new peak is attributed to Fe
2(SO
4)
3 [
37]. The formation of sulfate species is confirmed by the appearance of a new peak located at 167.9 eV in the S 2p spectrum in
Figure 2C and a new peak located at 532.2 eV in the O 1s spectrum in
Figure 2D [
36,
37]. This sulfate species might be formed as a result of the reaction between MBS and dissolved oxygen in the water, as shown by Equations (1) and (2).
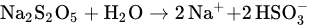
(1)
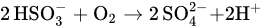
(2)
Figure 3 presents the X-ray photoelectron spectra of Mo 3d, S 2p, and O 1s of molybdenite. The Mo 3d spectra of the molybdenite surface (
Figure 3A) were best fitted with three GL functions. The 3d
5/2 peaks located at ca. 229.5 eV, 229.7 eV, and 232.2 eV are attributed to molybdenum bulk (Mo(IV)) of molybdenite [
40], Mo (IV) of molybdenum dioxide (MoO
2) [
41], and Mo(VI) of molybdenum trioxide (MoO
3) [
42], respectively. The S 2p spectrum of molybdenite shown in
Figure 3B indicates a doublet peak with S 2p
3/2 peaks located at 162.3 eV, which is attributed to the monosulfide of molybdenite [
19,
43].
Figure 3C shows the O 1s spectra of molybdenite. The presence of molybdenum dioxide and molybdenum trioxide is confirmed by the O 1s peaks located at 530.3 eV [
44] and 531.4 eV [
45], respectively. In addition, there are other peaks located at 532.3 eV and 533.7 eV, which are attributed to the hydroxide of the organic contaminants [
30] and adsorbed water (H
2O) [
39], respectively.
Figure 3. Mo 3d (A), S 2p (B), O 1s (C) spectra of chalcopyrite without and with 5 mM MBS treatment at pH 9.
Similar spectra of Mo 3d, S 2p, and O 1s of molybdenite were obtained after the molybdenite was treated in 5 mM MBS at pH 9. In addition, unlike the formation of sulfate species on the S 2p of chalcopyrite after being treated in 5 mM MBS at pH 9, there was no sulfate formation in the S 2p spectra of molybdenite.
4. Proposed Mechanism
Based on the XPS analysis results, a mechanism can be proposed to explain the depressing effect of MBS on the floatability of chalcopyrite. The MBS treatment mainly produces sulfate species as a reaction product when in contact with the dissolved oxygen in water (Equations (3) and (4)). This sulfate species covered the chalcopyrite surface, forming hydrophilic Fe2(SO4)3 species on the surface. Furthermore, the MBS treatment improved the intensity of hydrophilic FeOOH and Fe2O3 species on the chalcopyrite surface. The presence of these various hydrophilic species on the surface depresses the floatability of chalcopyrite.
The XPS results of molybdenite indicate that the MBS had an insignificant effect on the binding energy of chemical species on the surface of molybdenite. However, the flotation results indicate that MBS treatment exhibited a slightly depressing effect on the floatability of molybdenite. This difference in XPS and flotation results could be caused by the crystal structure of molybdenite. The crystal structure of molybdenite shows two types of surfaces: (1) non-polar faces formed by the scission of S–S bonds and (2) polar edges generated by the rupture of strong covalent Mo–S bonds. Based on these bonding types, the faces are hydrophobic and the edges are hydrophilic [
46]. These edges might be vulnerable to the MBS treatment than the faces as shown by Chen et al. [
32]. They reported that the MBS treatment showed an insignificant effect in the spectra of molybdenite faces, however, it formed sulfate and sulfite species in the S 2p spectra of molybdenite edges after being treated in 1 mM MBS in DI water.
In this study, the molybdenite fine powder was used for flotation tests and XPS analysis, which has a higher edges-to-faces ratio, thus the depressing effect of MBS on the floatability of molybdenite became more apparent. Furthermore, the molybdenite powder used for XPS analysis in this study was not differentiated between the faces and edges of molybdenite, thus the XPS analysis could not identify the effect of MBS treatment on the molybdenite edges. However, the presence of sulfate and sulfite species on the molybdenite edges, as reported by Chen et al. [
32], could explain the slightly depressing effect of MBS on the floatability of molybdenite presented in this study.
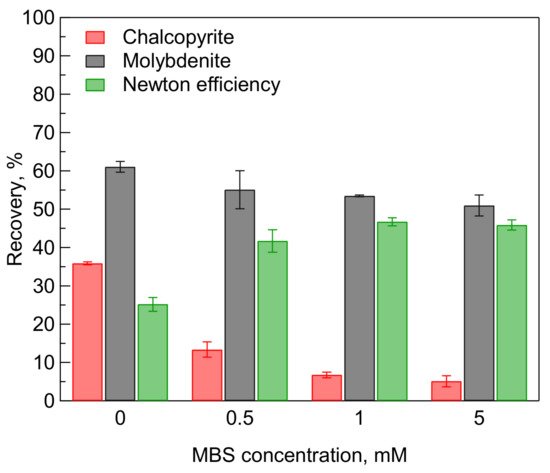
5. Microflotation of Mixed Minerals
The microflotation results of a single mineral, presented in Figure 1, show the possibility of using MBS treatment for selective separation of molybdenite and chalcopyrite. Therefore, the microflotation tests were performed using mixed chalcopyrite and molybdenite. Figure 4 shows the effects of MBS treatment on the recovery of chalcopyrite and molybdenite in the mixed minerals system.
Figure 4. Effect of sodium metabisulfite (MBS) on the recovery of mixed chalcopyrite and molybdenite (1:1 ratio) in the absence of a collector at pH 9.
As expected, the microflotation results of mixed chalcopyrite and molybdenite show a similar trend as shown in the microflotation of a single mineral (Figure 1). The MBS treatment exhibited a strong depressing effect on the recovery of chalcopyrite and slightly reduced the recovery of molybdenite. Furthermore, the depressing effect increased with an increase in the concentration of MBS.
The Newton efficiency is presented in Figure 4 to assess the selectivity of the MBS treatment on the separation of chalcopyrite and molybdenite using flotation. The Newton efficiency increased from 25% before the MBS treatment to 43% after the addition of 0.5 mM MBS. The Newton efficiency reached a maximum value (48%) after the addition of 1 mM MBS and then slightly decreased to 46% after the addition of 5 mM MBS owing to the decreasing recovery of molybdenite. These Newton efficiency results demonstrate that the MBS treatment could be used as a selective depressant of chalcopyrite in the selective flotation of chalcopyrite and molybdenite.
Emulsified kerosene and diesel oil were used as collectors to improve the separation selectivity of chalcopyrite and molybdenite in the flotation of mixed minerals using MBS treatment. These non-polar oil collectors were selected because they are known as selective collectors for molybdenite, owing to a higher affinity on the molybdenite non-polar faces [
2,
47,
48,
49,
50]. In these flotation tests, the concentration of MBS was fixed at 1 mM. This concentration was selected based on the maximum Newton efficiency shown in
Figure 4.
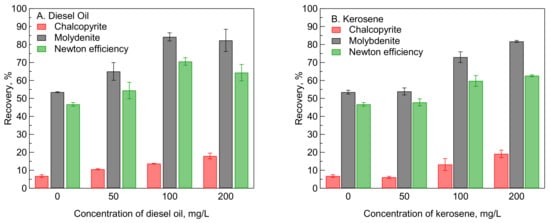
Figure 5 shows the effect of emulsified diesel oil and kerosene on the recovery of mixed chalcopyrite and molybdenite after being treated in 1 mM MBS at pH 9. The addition of various concentrations of emulsified diesel oil and kerosene significantly improved the recovery of molybdenite compared to that of chalcopyrite. For instance, the recovery of molybdenite significantly increased from 52% to 82% after the addition of 100 mg/L diesel oil, while the recovery of chalcopyrite slightly increased from 7% to 13% under a similar condition. These results can be attributed to the selective adsorption of diesel oil and kerosene on the molybdenite surface, which was less affected by the MBS treatment than the chalcopyrite surface, as shown by the XPS analysis and flotation results. In addition, Suyantara et al. [
48,
51] showed that the molybdenite surface became more hydrophobic after the addition of emulsified kerosene, thus molybdenite became more floatable compared to that of chalcopyrite.
Figure 5. Effect of emulsified diesel oil (A) and kerosene (B) on the recovery of mixed chalcopyrite and molybdenite after being treated in 1 mM MBS at pH 9.
Figure 5 demonstrates that emulsified diesel oil was more effective to improve the recovery of molybdenite compared to that of emulsified kerosene. For instance, the recovery of molybdenite improved from 52% to 82% after the addition of 100 mg/L emulsified diesel oil, while the recovery of molybdenite increased from 52% to 73% after the addition of 100 mg/L emulsified kerosene. Diesel oil has a better dispersion capability in water (i.e., forming finer and homogeneous emulsion) than kerosene [
52]; thus, it is more effectively adsorbed on the molybdenite surface.
Figure 5A indicates that the recovery of molybdenite reached an optimum condition after the addition of 100 mg/L emulsified diesel oil. The addition of a higher concentration (i.e., 200 mg/L) of emulsified diesel oil slightly reduced the recovery of molybdenite. One of the possible reasons for this phenomenon is the addition of excess emulsified diesel oil caused excessive agglomeration of molybdenite particles, causing the molybdenite particle to become too heavy to be carried by the nitrogen bubbles to the froth zone. On the other hand, this phenomenon was not observed if emulsified kerosene was used as a collector (Figure 5B). The maximum recovery of molybdenite was observed after the addition of 200 mg/L emulsified kerosene.
The recovery of chalcopyrite slightly increased with an increase in the concentration of emulsified diesel oil and kerosene (
Figure 5). This phenomenon can be caused by the physical adsorption of diesel oil and kerosene on the surface of chalcopyrite, owing to the presence of sulfur species on the slightly oxidized chalcopyrite, as shown from the XPS results. A similar phenomenon has been reported in previous studies [
48,
53]. In addition, this phenomenon might be caused by the interaction between molybdenite and chalcopyrite (i.e., heterocoagulation and entrapment of chalcopyrite), which requires further investigation. Indeed, Hornn et al. [
54] showed that emulsified kerosene can be used to agglomerate the fine particle chalcopyrite, improving its floatability in the agglomeration-flotation.
Table 1 confirms the effectiveness of MBS treatment as a selective depressant of chalcopyrite in the selective flotation of chalcopyrite and molybdenite with the addition of diesel oil and kerosene. In the absence of MBS treatment, the recoveries of chalcopyrite were 30.8% and 43.3% with the addition of 100 mg/L diesel oil and 200 mg/L kerosene, respectively. Meanwhile, the addition of diesel oil and kerosene significantly improved the recoveries of molybdenite, from 61.1% in the absence of collector to 88.0% and 89.5% with the addition of 100 mg/L diesel oil and 200 mg/L kerosene, respectively. With the addition of 1 mM MBS, the recovery of chalcopyrite significantly depressed to 13.7% and 19.1% under similar conditions. On the other hand, the recovery of molybdenite slightly decreased after the MBS treatment.
Table 1. Recovery of chalcopyrite (Cu) and molybdenite (Mo) and Newton efficiency (η) under the optimal condition of diesel oil and kerosene.
| No |
Collector Condition |
MBS Concentration |
Recovery, % |
η, % |
| Cu |
Mo |
| 1 |
Without collector |
0 mM MBS |
35.9 |
61.1 |
25.2 |
| 2 |
100 mg/L diesel |
0 mM MBS |
30.8 |
88.0 |
57.3 |
| 3 |
100 mg/L diesel |
1 mM MBS |
13.7 |
84.2 |
70.5 |
| 4 |
200 mg/L kerosene |
0 mM MBS |
43.3 |
89.5 |
46.2 |
| 5 |
200 mg/L kerosene |
1 mM MBS |
19.1 |
81.7 |
62.6 |
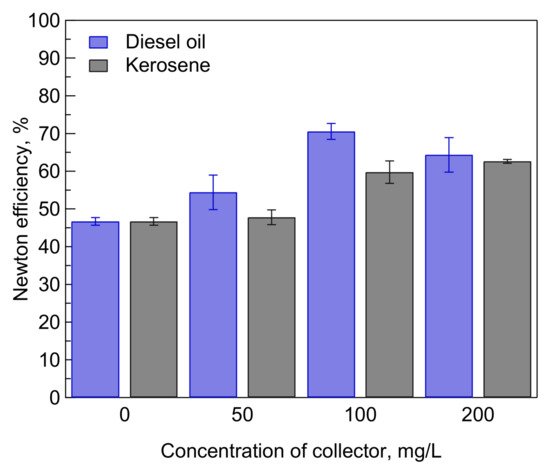
Figure 6 shows the Newton efficiency of flotation of mixed chalcopyrite and molybdenite after being treated in various concentrations of emulsified diesel oil and kerosene in 1 mM MBS at pH 9. The Newton efficiency gradually increased with an increase in the concentration of emulsified diesel oil and kerosene. The Newton efficiency reached a maximum value of 70% after the addition of 100 mg/L diesel oil in 1 mM MBS at pH 9. Under a similar concentration, the addition of emulsified kerosene results in lower Newton efficiency (i.e., 60%). Meanwhile, the Newton efficiency reached a maximum value of 62% after the addition of 200 mg/L emulsified kerosene in 1 mM MBS at pH 9. This result indicates that emulsified diesel oil is more effective in improving the selectivity of flotation of chalcopyrite and molybdenite compared to kerosene. As discussed previously, diesel has a higher effectivity owing to it having a better dispersion capability in water than kerosene. Nevertheless, both emulsified diesel oil and kerosene could significantly improve the separation selectivity of selective flotation of chalcopyrite and molybdenite using MBS treatment.
Figure 6. Effects of diesel oil and kerosene addition on the Newton efficiency of selective flotation of chalcopyrite and molybdenite in 1 mM MBS at pH 9.
This entry is adapted from the peer-reviewed paper 10.3390/min11121377
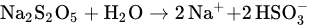 (1)
(1)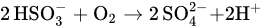 (2)
(2)