Stink bugs use semiochemicals to communicate over long distances and exchange vibratory signals that are transmitted on plants over shorter distances. These signals are produced by various mechanisms, such as body vibration (tremulation) or drumming on the substrate, and are accompanied by visual, chemical, and mechanical signals and cues when they encounter a mate.
- plant-dwelling insects
- biotremology
- host plants
- sexual communication
- chemical signals
- vibratory signals
1. Communication of Stink Bugs during Calling, Courtship and Rivalry
In many stink bugs, communication begins with the emission of male species-specific pheromones, which attract females from longer distances [1] (Figure 1). Once on the same plant, information exchange between stink bugs takes place via substrate-borne signals [1][2][3]. Insects most frequently use vibrations as the means of communication on plants or other substrates [4]. Studies on stink bugs, and many other insects [5][6][7][8][9] have described various behavioral and neural mechanisms involved in substrate-borne vibratory communication.
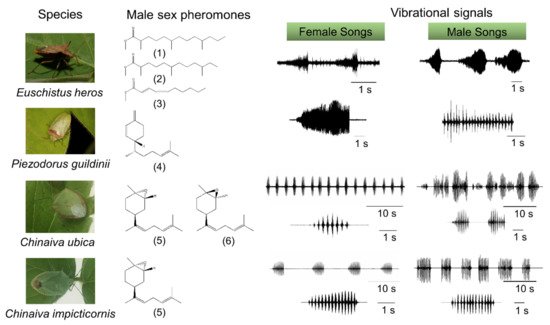
1.1 Vibrational communication
In the southern green stink bug, Nezara viridula (Pentatomidae), a male searches for a mate and emits calling and courtship substrate-borne signals in response to vibratory calls from the stationary female [5][6][7]. In both sexes, these signals are produced by abdominal vibration (AV signals). Many stink bugs form a stereotyped female-male duet that is crucial for mate finding and recognition [10][11][12][13][14][15]. Upon encountering a mate, the vibratory calling phase transitions into a multimodal courtship phase in which AV signals are accompanied by visual and tactile signals and cues, the latter acting through both chemical and mechanical channels [5][6][7]. The exchange of calling and courtship signals between mates is inhibited by male or female rivalry when multiple individuals compete for copulation with the same mate [5][16][17].
The AV signals have been described in 36 stink bug species as components of calling, courtship, rivalry, and copulation songs [5]. They share a common fundamental (basic) frequency that typically ranges between 90 and 120 Hz (Figure 2), with the extreme lowest and highest values measured to date in N. antennata (50 Hz, female calling song) and Euschistus heros (175 Hz, male courtship song) [18][19]. AV signal amplitudes (expressed in velocity units) range from 0.1 to 1 mm/s when measured on the body of emitter bugs standing on a plant [20].Among the different types of vibratory signals, the AV signals show the greatest diversity of temporal characteristics that determine their species and sex specificity. The temporal pattern of elements of the same song type can differ considerably among populations of the same species, as shown, for example, in N. viridula from Brazil, Florida, Italy, and Slovenia [21]. Accordingly, genetic differences have been described between 11 geographically separated populations of N. viridula from Europe (Slovenia, France, Greece, Italy and Madeira), Japan, Guadalupe, Galapagos, California, Brazil and Botswana [22]. Several playback experiments have confirmed that the duration of song components and the intervals between them mediate crucial information for recognition and directionality[10][14][15][23](Figure 2).
The temporal pattern of calling, courtship, and rivalry songs determines their function in communication [13]. The female calling song is characterized by longer periods of signals with a simple repetitive pattern, enabling directional movement of the male towards the calling mate. The courtship song has a more complex structure, and is supported by signals from other modalities that enhance recognition and motivate the mates to copulate. Male rivalry song of most species studied to date is characterized by a short-term production of rapidly repeated shorter and often frequency-modulated (FM) pulses. Typically, following signal alternation, one of the rivals stops signaling [13][14][15]. Female rivalry is more complex and involves prolonged alternation with the rival signals and modified calling signals [24][25]. In addition, vibrations of the raised wings (buzzing signals) or whole body tremulation have been described as constituents of communication in E. heros, C. impicticornis and C. ubica. These signals are of high amplitude, broad-band nature, and are species- but not sex-specific [26][27]. Buzzing signals usually precede AV signals in the very early phase of communication [27], while whole body tremulation has been observed in the context of aggression (rivalry) and shortly preceding copulation [26]. Moreover, stink bugs produce low-amplitude percussion signals by tapping the substrate with their forelegs. These signals are species- and sex-specific and occur in a variety of contexts, for example, immediately after copulation [5], but the function of these signals has largely not been elucidated.
1.2 Chemical communication
2. Transmission of Vibratory Signals through Plants
3. The Effect of Vibratory Noise on Communication
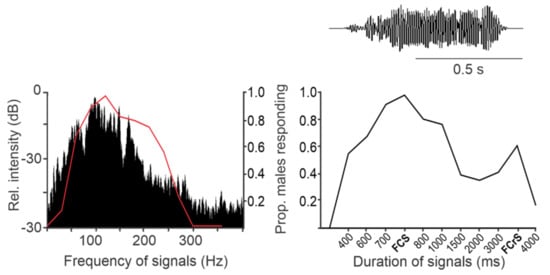
Since stink bug vibratory signals are narrow-banded, the sensory system can selectively filter out noise of lower and higher frequencies without affecting signal information. At the same time, species-specificity of signals is not encoded in their frequency domain, consequently implying larger heterospecific overlap [e.g., [48] [49].
Wind and rain represent the main source of noise in the field, characterized by most energy content at very low frequencies (below 30 Hz) [50][51][52][53][54][55][56][57]. These frequencies lie below that characteristic of stink bug AV signals and the peak sensitivity of specialized vibratory receptor organs [58]. Nevertheless, in laboratory experiments, significantly lower copulatory success was observed in E. heros stink bugs exposed to airflow or rain-generated vibratory noise [25]. Additional experiments are needed to test the influence of low-velocity wind on vibrational communication of stink bugs in the field.
Vibratory noise has a number of effects on stink bug communication [17][59][60][61]. In N. viridula, for example, exposure of male-female pairs to pure-tone background vibrations with frequency and amplitude characteristics similar to those of conspecific signals had no effect on the expression of male searching behavior. Females, on the other hand, discontinued their calls, reduced the repetition rate of their emissions, or replaced their calls with rival songs. Furthermore, females changed the fundamental frequency of the calling song so to increase its differentiation from the background vibration [59]. Similarly, males and females of E. heros adjusted the repetition rate of their calls and modified their fundamental frequency in response to vibratory noise [41]. These modifications can all be interpreted as the mechanisms for reducing signal masking interference. Rival songs of C. impicticornis, C. ubica, and N. viridula act in a similar way, as they inhibit simultaneous singing by multiple mates [13][14][15]. In contrast, in N. viridula, low-intensity white Gaussian noise presented on a plant along with subthreshold female calling song positively affects male searching behaviour. The intensity-response characteristics of this influence suggest that it is due to the stochastic threshold resonance [62].
This entry is adapted from the peer-reviewed paper 10.3390/insects12121058
References
- Michelsen, A.; Fink, F.; Gogala, M.; Traue, D. Plants as transmission channels for insect vibrational songs. Behav. Ecol. Sociobiol. 1982, 11, 269–281.
- Čokl, A.; Blassioli-Moraes, M.C.; Laumann, R.A.; Žunič, A.; Borges, M. Stinkbugs—multisensory communication with chemical and vibratory signals transmitted through different media. In Biotremology—Studying Vibrational Behavior, 1st ed.; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 91–122.
- Čokl, A.; Žunič-Kosi, A.; Laumann, R.A. Stink Bug Communication with Multimodal Signals Transmitted through Air and Substrate. Emerg. Sci. J. 2019, 3, 407–424.
- Čokl, A.; Kosi, A.; Žunič, A.; Laumann, R.A.; Doberlet, M.V. Female competition for availability of males in insects: The Nezara viridula (Linnaeus, 1758) model. Insect Sci. 2020, 27, 801–814.
- Dias, A.; Borges, M.; Moraes, M.B.; Coelho, M.L.F.; Čokl, A.; Laumann, R. Inhibitory Copulation Effect of Vibrational Rival Female Signals of Three Stink Bug Species as a Tool for Mating Disruption. Insects 2021, 12, 177.
- Čokl, A.; Laumann, R.; Kosi Žunič, A.; Blassioli-Moraes, M.C.; Doberlet, M.V.; Borges, M. Interference of Overlapping Insect Vibratory Communication Signals: An Eushistus heros Model. PLoS ONE 2015, 10, e0130775.
- de Groot, M.; Čokl, A.; Doberlet, M.V. Effects of heterospecific and conspecific vibrational signal overlap and signal-to-noise ratio on male responsiveness in Nezara viridula (L.). J. Exp. Biol. 2010, 213, 3213–3222.
- Cocroft, R.B.; Rodriguez, R.L. The behavioral ecology of insect vibrational communication. Bioscience 2005, 55, 323–334.
- Casas, J.; Bacher, S.; Tautz, J.; Meyhöfer, R.; Pierre, D. Leaf Vibrations and Air Movements in a Leafminer–Parasitoid System. Biol. Control. 1998, 11, 147–153.
- Barth, F.G. The vibrational sense in spiders. In Handbook of Auditory Research: Insects, 1st ed.; Hoy, R.R., Popper, R.R., Fay, A.N., Eds.; Springer: New York, NY, USA, 1988; pp. 228–278. ISBN 978-1-4612-0585-2.
- McVean, A.; Field, L.H. Communication by substratum vibration in the New Zealand tree weta, Hemideina femorata (Stenopelmatidae: Orthoptera). J. Zoöl. 1996, 239, 101–122.
- Tishechkin, D. Vibratory communication in Psylloidea (Hemiptera). In Insect Sounds and Vibration: Physiology, Behaviour, Ecology and Evolution, 1st ed.; Drosopoulos, S., Claridge, M.F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 357–363. ISBN 0-84963-2060-7.
- McNett, G.D.; Cocroft, R.B. Host shifts favor vibrational signal divergence in Enchenopa binotata treehoppers. Behav. Ecol. 2008, 19, 650–656.
- Šturm, R.; Polajnar, J.; Doberlet, M.V. Practical Issues in Studying Natural Vibroscape and Biotic Noise. In Coding Strategies in Vertebrate Acoustic Communication; Springer: Singapore, 2019; pp. 125–148.
- Velilla, E.; Muñoz, M.; Quiroga, N.; Symes, L.; Ter Hofstede, H.M.; Page, R.A.; Simon, R.; Ellers, J.; Halfwerk, W. Gone with the wind: Is signal timing in a neotropical katydid an adaptive response to variation in wind-induced vibratory noise? Behav. Ecol. Sociobiol. 2020, 74, 1–11.
- Čokl, A. Functional poperties of viboreceptors in the legs of Nezara viridula (L.) (Heteroptera, Pentatomidae). J. Comp. Physiol. A 1983, 150, 261–269.
- Spezia, S.; Curcio, L.; Fiasconaro, A.; Pizzolato, N.; Valenti, D.; Spagnolo, B.; Bue, P.L.; Peri, E.; Colazza, S. Evidence of stochastic resonance in the mating behavior of Nezara viridula (L.). Eur. Phys. J. B 2008, 65, 453–458.
- Kon, M.; Oe, A.; Numata, H.; Hidaka, T. Comparison of the mating behaviour between two sympatric species, Nezara antennata and N. viridula (Heteroptera: Pentatomidae), with special reference to sound emission. J. Ethol. 1988, 6, 91–98.
- Blassioli-Moraes, M.C.; Laumann, R.A.; Čokl, A. Vibratory signals of four Neotropical stink bug species. Physiol. Entomol. 2005, 30, 175–188.
- Čokl, A.; Zorović, M.; Millar, J.G. Vibrational communication along plants by the stink bugs Nezara viridula and Murgantia histrionica. Behav. Process. 2007, 75, 40–54.
- Cˇokl, A.; Virant-Doberlet, M.; Stritih, N. The structure and function of songs emitted by southern green stink bugs from Brazil, Florida, Italy and Slovenia. Physiol. Èntomol. 2000, 25, 196–205.
- Kavar, T.; Pavlovčič, P.; Sušnik, S.; Meglič, V.; Virant-Doberlet, M. Genetic differentiation of geographically separated populations of the southern green stink bug Nezara viridula (Hemiptera: Pentatomidae). Bull. Èntomol. Res. 2006, 96, 117–128.
- Hrabar, N.; Virant-Doberlet, M.; Čokl, A. Species specificity of male southern green stink bug Nezara viridula (L.) reactions to the female calling song. Acta Zool. Sinica. 2004, 50, 566–575.
- Kavčič, A.; Čokl, A.; Laumann, R.A.; Blassioli-Moraes, M.C.; Borges, M. Tremulatory and Abdomen Vibration Signals Enable Communication through Air in the Stink Bug Euschistus heros. PLoS ONE 2013, 8, e56503.
- Čokl, A.; Kosi Žunič, A.; Moraes, M.C.B.; Borges, M.; Laumann, R.A. Stink Bug Inter-Plant Communication with Signals Produced by Vibration of Lifted Wings. J. Insect Behav. 2021, 34, 194–210.
- Zgonik, V.; Čokl, A. The role of signals of different modalities in initiating vibratory communication in Nezara viridula. Open Life Sci. 2014, 9, 200–211.
- McBrien, H.L.; Millar, J.G. Substrate-borne vibrational signals of the Consperse stink bug (Hemiptera: Pentatomidae). Can. Èntomol. 2003, 135, 555–567.
- Colazza, S.; Aquila, G.; De Pasquale, C.; Peri, E.; Millar, J.G. The egg parasitoids Trissolcus basalis uses n-nonadecane, a cuticular hydrocarbon from its stink bug host Nezara viridula, to discriminate between female and male hosts. J. Chem. Ecol. 2007, 33, 1405–1420.
- Silveira, S. Isolamento Reprodutivo em Duas Espécies Simpátricas de Chinavia (Orian (Hemiptera: Pentatomidae): Importância da Comunicação Vibracional e Composição Química da Cutícula. Master’s Thesis, Universidade de Brasília, Brasilia, Brazil, 2015.
- Guarino, S.; De Pasquale, C.; Peri, E.; Alonzo, G.; Colazza, S. Role of volatile and contact pheromones in the mating behaviour of Bagrada hilaris (Heteroptera: Pentatomidae). Eur. J. Èntomol. 2008, 105, 613–617.
- Colazza, S.; Bue, M.L.; Giudice, D.L.; Peri, E. The response of Trissolcus basalis to footprint contact kairomones from Nezara viridula females is mediated by leaf epicuticular waxes. Naturwissenschaften 2009, 96, 975–981.
- Cremer, L.; Heckl, M. Structure-Borne Sound; Springer: Berlin/Heidelberg, Germany, 1973; p. 528.
- Polajnar, J.; Svenšek, D.; Čokl, A. Resonance in herbaceous plant stems as a factor in vibrational communication of pentatomide bugs (Heteroptera: Pentatomidae). J. Roy. Soc. Interf. 2012, 9, 1898–1907.
- Cokl, A.; Presern, J.; Virant-Doberlet, M.; Bagwell, G.J.; Millar, J.G. Vibratory signals of the harlequin bug and their transmission through plants. Physiol. Èntomol. 2004, 29, 372–380.
- Cokl, A.; Žunič, A.; Millar, J. Transmission of Podisus maculiventris tremulatory signals through plants. Open Life Sci. 2009, 4, 585–594.
- Žunič, A.; Čokl, A. Predatory Stink Bugs (Asopinae) and the Role of Substrate-borne Vibrational Signals in Intra- and Interspecific Interactions. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 59–77.
- Gogala, M.; Razpotnik, R. A method of oscillographic sonagraphy for bio-acoustic. Res. Biol. Vestnik. 1974, 22, 209–216.
- Čokl, A.; Laumann, R.; Kosi Žunič, A.; Blassioli-Moraes, M.C.; Doberlet, M.V.; Borges, M. Interference of Overlapping Insect Vibratory Communication Signals: An Eushistus heros Model. PLoS ONE 2015, 10, e0130775.
- Polajnar, J.; Eriksson, A.; Doberlet, M.V.; Mazzoni, V. Mating disruption of a grapevine pest using mechanical vibrations: From laboratory to the field. J. Pest Sci. 2016, 89, 909–921.
- Čokl, A.; Nardi, C.; Bento, J.M.S.; Hirose, E.; Panizzi, A.R. Transmission of stridulatory signals of the burrower bugs, Scaptocoris castanea and Scaptocoris carvalhoi (Heteroptera: Cydnidae) through the soil and soybean. Physiol. Èntomol. 2006, 31, 371–381.
- Michelsen, A.; Fink, F.; Gogala, M.; Traue, D. Plants as transmission channels for insect vibrational songs. Behav. Ecol. Sociobiol. 1982, 11, 269–281.
- Čokl, A. Vibratory signal transmission in plants as measured by laser vibrometry. Period. Biol. 1988, 90, 193–196.
- Koczor, S.; Čokl, A. Percussion signals of Lygus rugulipennis Poppius (Heteroptera: Miridae). Open Life Sci. 2014, 9, 543–549.
- Miklas, N.; Stritih, N.; Čokl, A.; Virant-Doberlet, M.; Renou, M. The Influence of Substrate on Male Responsiveness to the Female Calling Song in Nezara viridula. J. Insect Behav. 2001, 14, 313–332.
- Polajnar, J.; Čokl, A. The effect of vibratory disturbance on sexual behaviour of the southern green stink bug Nezara viridula (Heteroptera, Pentatomidae). Open Life Sci. 2008, 3, 189–197.
- Laumann, R.A.; Maccagnan, D.H.B.; Čokl, A.; Blassioli-Moraes, M.C.; Borges, M. Substrate-borne vibrations disrupt the mating behaviors of the neotropical brown stink bug, Euschistus heros: Implications for pest management. J. Pest Sci. 2018, 91, 995–1004.
- Spezia, S.; Curcio, L.; Fiasconaro, A.; Pizzolato, N.; Valenti, D.; Spagnolo, B.; Bue, P.L.; Peri, E.; Colazza, S. Evidence of stochastic resonance in the mating behavior of Nezara viridula (L.). Eur. Phys. J. B 2008, 65, 453–458.
