The syntheses of the title compounds demonstrate a privileged introduction of a nitroso (and a hydroxyl via the Baudisch reaction) group to an aromatic ring. These complexes first appeared in the literature as early as 1939, and a range of applications has subsequently been published. However, optimisations of the preparative sequences were not considered, and as such, the reactions have seldom been utilised in recent years; indeed, there remains confusion in the literature as to how such complexes form.
- C-nitrosation
- copper complexes
- Baudisch reaction
- nitrosophenols
- ortho-nitrosophenols
- regioselective aromatic functionalisation
1. Introduction to Metal-Nitrosophenolato Complexes
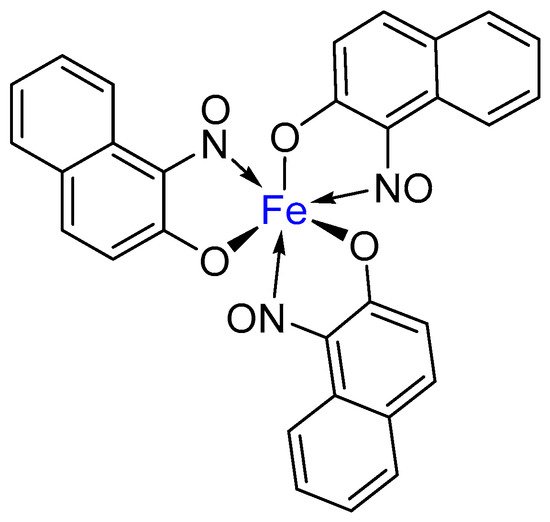
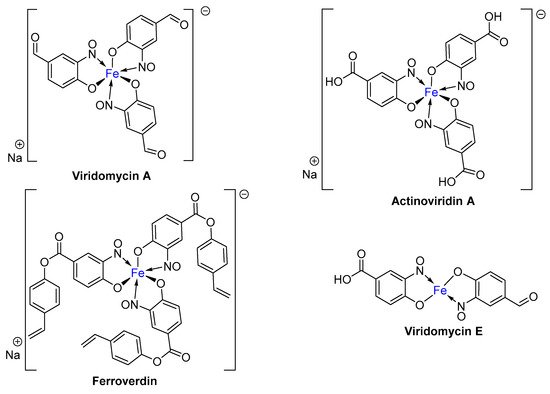
2. Metal-Nitrosophenolato Complexes in Nature
3. A Brief History of Metal-Nitrosophenolato Synthesis
4. Introduction to the Baudisch Reaction
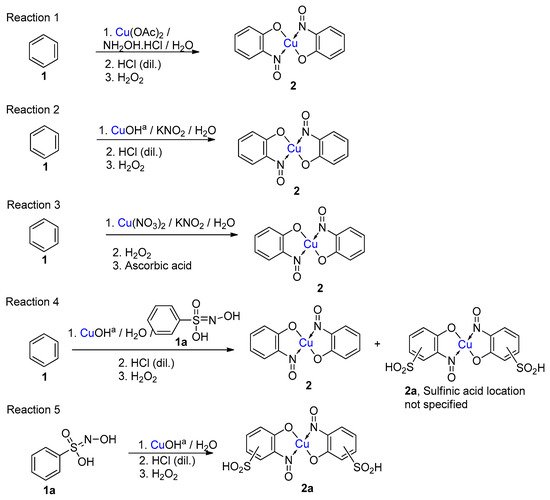
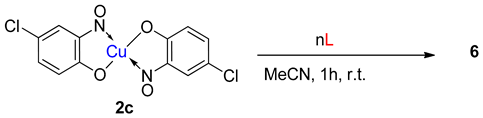
5. Copper-Mediated Aromatic Nitrosation
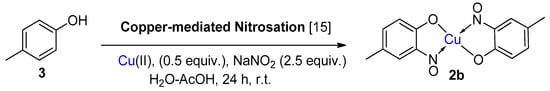
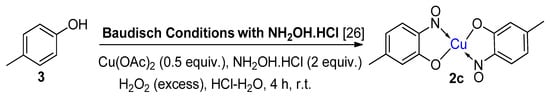
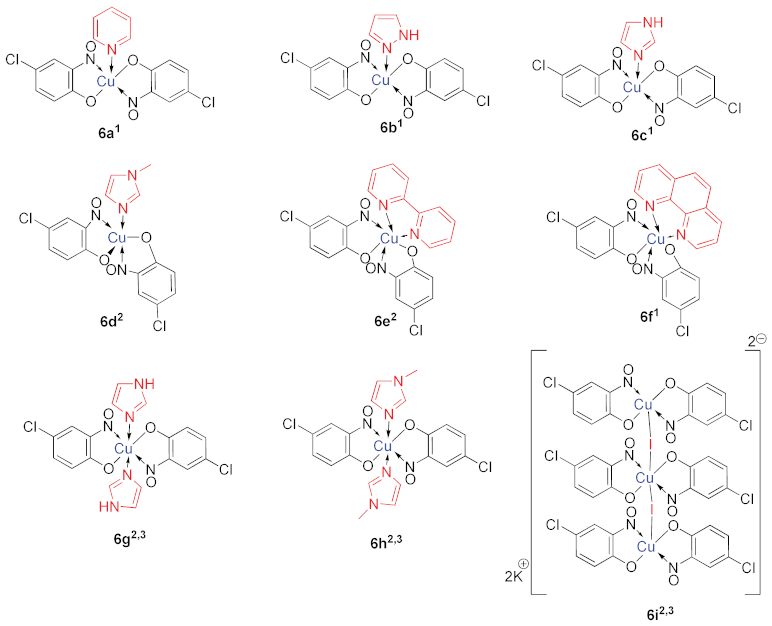
6. Additional Syntheses of Metal-Nitrosophenolato Complexes
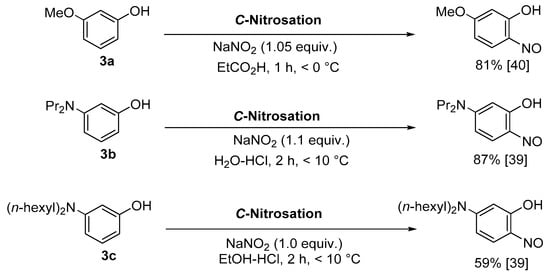
|
Description |
Accepted Starting Materials |
|---|---|
|
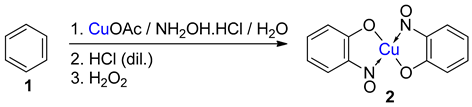
Baudisch conditions with hydroxylamine |
A range of aromatics including benzene, phenols, catechols, naphthalenes and phenylsulfinic acids [20,42,43]. |
|
|
|
|
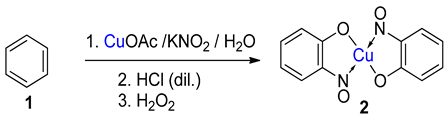
Baudisch conditions with nitrous acid |
Shown to accept benzene. Similar aromatics expected to work, though scope not investigated [20]. |
|
|
|
|
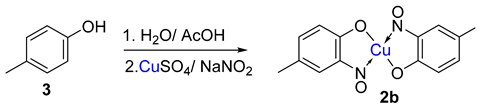
Copper-mediated aromatic nitrosation |
Phenols with sufficiently electron-rich aromatic ring and at least one non-functionalised carbon atom ortho to the phenol [15,41]. |
|
|
|
|
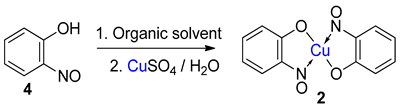
Association of free 2-nitrosophenols with copper |
|
|
|
7. Scope of Metal-Nitrosophenolato Synthesis
8. Properties of Metal-2-Nitrosophenolato Complexes
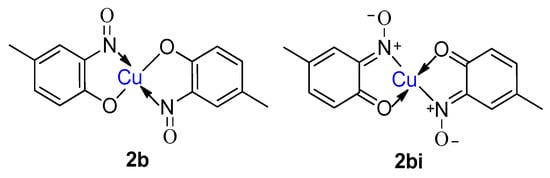
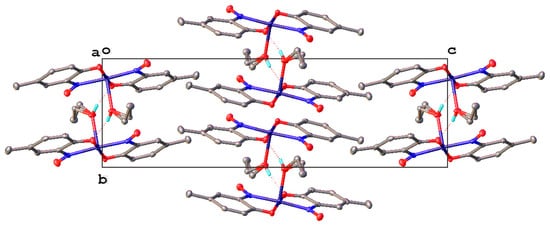
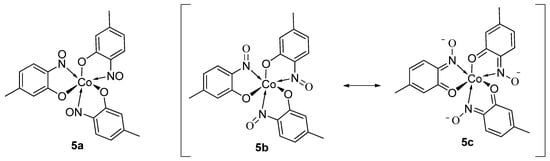
9. Derivatisation of Complexes
10. Conclusion
This entry is adapted from the peer-reviewed paper 10.3390/molecules24224018