Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Antibiotic-associated diarrhea (AAD) is a self-limiting disease mediated by antibiotic therapy. In clinical practice, several types of probiotics are used in treating AAD, but minimal research has been done on Bacteroides-based microecologics.
- Bacteroides
- Bifidobacterium
- AAD
1. Introduction
Antibiotic-associated diarrhea (AAD) is a major clinical complication caused by side effects and the overuse of antibiotics [1][2]. AAD is estimated to typically occur in approximately 5–30% of patients during or at the end of antibiotic therapy [3], and often caused significant changes in gut microbiota [4]. Clinical symptoms in patients with AAD vary from mild diarrhea without complications to severe colitis, fulminant pseudomembranous colitis, or even death [5]. A possible mechanism of AAD is the antibiotic acting directly on the intestinal mucosa, thereby leading to the overgrowth of pathogenic bacteria such as Clostridium difficile Staphylococcus, Candida, Enterobacteriaceae, and Klebsiella [6]. Of these, C. difficile is the most common cause of AAD infections [7][8]. The use of antibiotics can cause dysregulation of the metabolic activity of colonic microbiota [9]. Microbial metabolites can modulate the metabolic integrity of epithelial cells and elicit immune reactions [10][11]. In addition, decreased resistance to pathogens caused by AAD is associated with alterations in the metabolism of carbohydrates, SCFAs, and bile acids [12].
Growing evidence indicates that AAD is caused by dysbiosis of the gut microbiota caused by antibiotic treatment [13][14]. Probiotics may maintain or recover intestinal microecology through nutritional competition, receptor competition, favoring the growth of nonpathogenic bacteria, inhibiting mucosal adhesion of pathogens, or simulating immunity during or after antibiotic treatment [15][16]. Current research is mainly focused on the genera Lactobacillus, Bifidobacterium, and Saccharomyces, which are commonly used to prevent or treat AAD [17]. Bifidobacterium preparations are increasingly being used in treating pediatric AAD. Pooled evidence from a systematic review, including five Bifidobacterium preparations from 30 trials, showed that Bifidobacterium preparations might be effective in preventing and treating pediatric AAD [18]. In addition, a study showed that long-term consumption of Clostridium butyricum in combination with a B. infantis mixture facilitated the recovery of gut microbiota and colonic tissue structure, which was a superior response to that observed in single strains in AAD treatments [19]. All of these studies have reinforced the potential role of probiotics in alleviating AAD.
Bacteroides are being actively researched as next-generation probiotics (NGPs) because of their potential benefits to human health. Some of these species can inhibit pathogenic bacteria colonization [20] and alleviate intestinal inflammation [21]. In a recent study, researchers determined that B. fragilis ZY -312 recovered epithelial cell organization and barrier functions through the ERK signaling pathway, thereby improving the abundance of specific commensal microbiota, and subsequently ameliorating diarrheal symptoms associated with AAD [22]. Moreover, the latest study correlated data from 117 individuals in four population-based cohorts and found that 21 bacteria species, including Bacteroides intestinalis, B. uniformis, B. adolescentis, and B. bifidum, showed a strong correlation with ecological recovery following antibiotic treatment [23]. In addition, a series of studies have demonstrated that B. uniformis can reduce the expression of acyl carrier proteins, thereby inhibiting increases in lipopolysaccharide (LPS)-induced proinflammatory cytokine levels, and beneficially reducing inflammation [24][25].
2. Effects of Different Treatments on Diarrhea Status Scores and Water Content in Feces
Initially, after the administration of lincomycin hydrochloride, all mice except the control group showed mental depression, a red anus, and diarrhea. Compared with the control group, the diarrhea status scores and stool water content of the model group was significant, which suggested that the AAD mouse model was successfully established. As shown in Figure 1B, a significant decrease in diarrhea status scores was observed in the Bu, Bi + Ba, and Bu + Ba groups. Among them, the Bu + Ba group showed a better result than the Bu group. Furthermore, the Bi + Ba and Bu + Ba groups exhibited significantly reduced stool water content. Although the Bi or Bu groups also showed a significant decrease, the effect was not as great as the mixed bacteria group (Figure 1C).
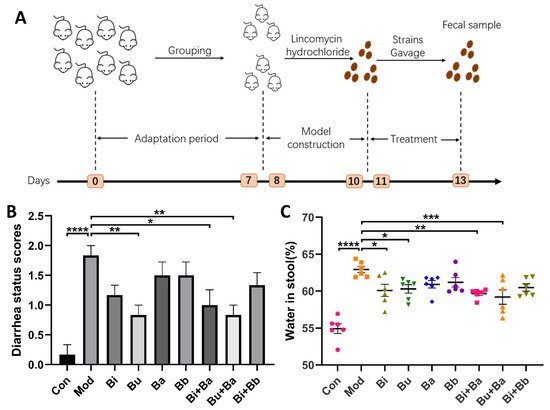
Figure 1. (A) Schematic diagram of the experimental design: (B) diarrhea status scores; and (C) water content in stool. *, **, ***, and **** indicate significant differences (p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively) between groups.
3. Effect of Different Treatments on Histopathological Structure of Colon Tissues
As shown in Figure 2, the colons of mice in the control group exhibited normal histological features. The mucosal epithelium remained intact, and the intestinal glands were abundant and closely arranged. Compared with the control group, the colonic mucosa of AAD mice showed a small inflammatory cell infiltration and mild edema in the submucosa. There was a slight reduction in the number of cupped glandular cells. These pathological characteristics were also observed in most treatment groups. However, no significant histopathological lesions were observed in mice treated with Bu or Bu + Ba, thereby indicating that it may reduce intestinal inflammation.
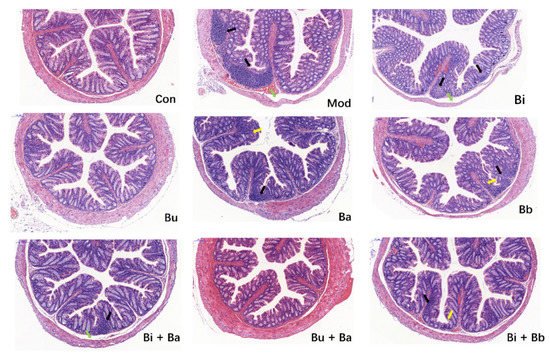
Figure 2. Histological examination of the representative image of H&E staining (scale bar = 100 µm). Yellow arrow—depletion of goblet cells; green arrow—mucosal edema; and black arrow—inflammatory cellular infiltration.
4. Effects of Different Treatments on Proinflammatory Cytokine Expression
To compare the immunomodulatory effects of different strains on AAD mice, cytokines were detected in colonic tissues by ELISA (Figure 3). The levels of IL-6 in the model group were significantly higher than those in the control group. Among the tested strains, the Bu + Ba group showed reduced levels of IL-6. Although the levels of IL-1β, IL-17, and TNF-α in the colonic tissues of mice were not significantly different from those in the control group, they showed a modest increase, whereas the Bu + Ba treatment decreased these proinflammatory inflammatory factors.
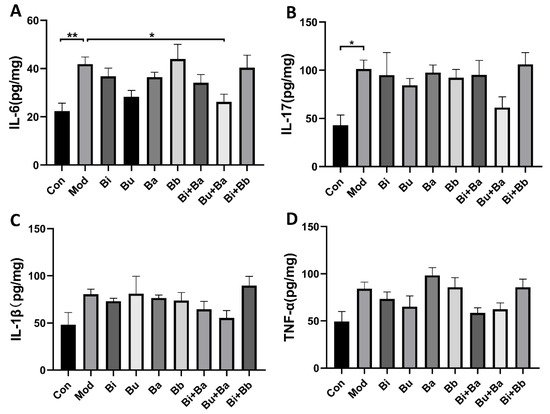
Figure 3. Analysis of cytokines in colon tissue by ELISA: (A) IL-6, (B) IL-17, (C) IL-1β, and (D) TNF-α. * and ** indicate significant differences (p < 0.05, p < 0.01 respectively) between groups.
5. Effects of Different Treatments on Intestinal Barrier Integrity
To analyze the regulation of different treatments on the intestinal barrier, serum LPS levels were determined. As shown in Figure 4, the level of LPS was significantly higher in the model group, whereas the Bi + Ba and Bi + Bb groups showed decreased LPS levels in the serum. Moreover, the Bi + Ba group showed a significant difference compared with the model group. Compared with the control group, the mRNA expression of occludin and Mucin-2 significantly decreased in the model group. However, the Bu + Ba treatment enhanced the expression of occludin in the colon to the level of the control group. In addition, Bi + Ba and Bu + Ba also increased the Mucin-2 expression. In the model group, diarrhea induced a significant decrease in the expression of NHE3 in the colonic tissues of the mice, whereas a supplementation with Bi, Bi + Ba, and Bu + Ba significantly increased this expression up to the level of the control group.
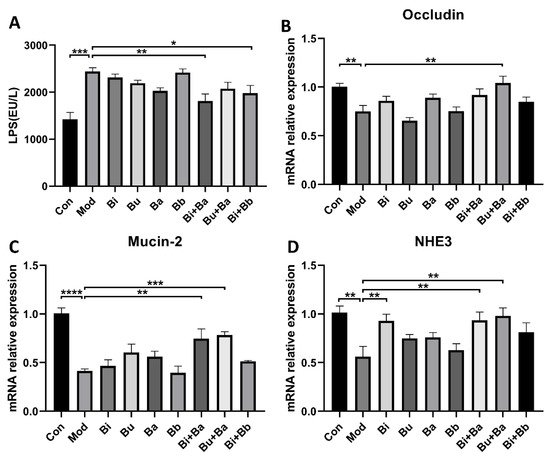
Figure 4. ELISA analyses of (A) lipopolysaccharide (LPS). Real-time PCR analysis of (B) occludin, (C) Mucin-2, and (D) NHE3, mRNA expression normalized to β-actin in the colons. *, **, ***, and **** indicate significant differences (p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively) between groups.
6. Effects of Different Treatments on SCFA Production
After treatment with lincomycin hydrochloride, the concentrations of acetic acid, propionic acid, isobutyric acid, and isovaleric acid in the caecal specimens of the model group mice were significantly reduced (Figure 5). Compared with the model group, the Bi and Bu groups had significantly increased acetic acid, propionic acid, and isobutyric acid concentrations. The Bi + Ba treatment significantly increased propionic acid, isobutyric acid, and isovaleric acid, but did not significantly increase the level of acetic acid. Significant increases in acetic acid, propionic acid, isobutyric acid, and isovaleric acid concentrations were also observed in the Bu + Ba group. The Bi + Bb group showed significantly increased levels of propionic acid, isobutyric acid, and isovaleric acid in the caecal specimens. These results also indicated that the gavage of Bacteroides and the mixture of probiotics most strongly benefitted the recovery of SCFA concentrations in AAD mice.
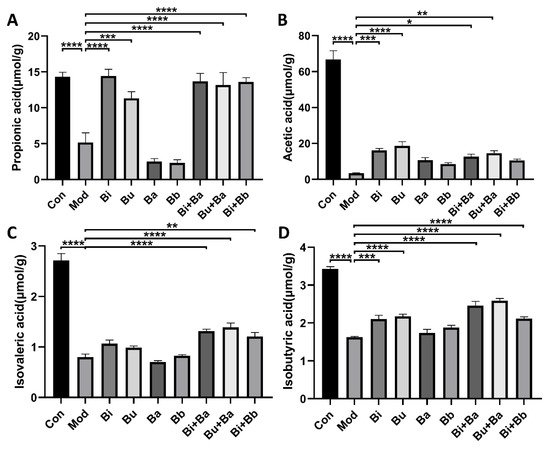
Figure 5. Effects of different treatments on the concentrations of (A) propionic acid, (B) acetic acid, (C) isovaleric acid, and (D) isobutyric acid. *, **, ***, and **** indicate significant differences (p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively) between groups.
7. Effect of Different Treatments on the Composition and Diversity of Gut Microbiota
The evenness and Shannon indexes presented the evenness and diversity of the gut microbiota, respectively. The results showed that the evenness and diversity were significantly reduced following the lincomycin hydrochloride treatment (Figure 6A,B). Compared with the model group, the Bi + Ba, Bu + Ba, and Bi + Bb groups showed increased evenness index, and there was a significant difference in the increase in the Bu + Ba group. The highest Shannon index values were observed in the Bi + Ba and Bu + Ba groups. The Bi and Bi + Bb groups also exhibited a significant increase. The purpose of the β-diversity analysis was to determine the similarities among the groups, and the principal component analysis (PCoA) of community β-diversity and clustering of distance based on the Jaccard index analysis indicated that the clusters of the microbial compositions of the Bi, Bu, Bi + Ba, Bu + Ba, and Bi + Bb groups were closer to the control group than the model group; this indicated that all these treatment groups potently restored the intestinal microbiota disorder (Figure 6C,D). In conclusion, the mixture of Bacteroides and Bifidobacterium most positively impacted the recovery of flora diversity in AAD mice, with the Bu + Ba group facilitating the best recovery rate.
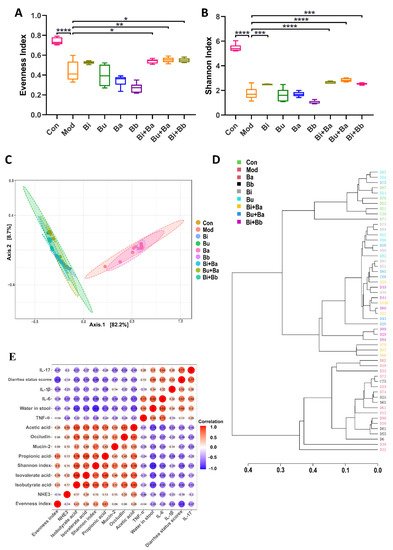
Figure 6. (A) Evenness index, (B) Shannon index, (C) principal coordinate analysis (PCoA), (D) clustering of distance based on the Jaccard index, and (E) correlation between the physiological and anti-inflammatory effects. *, **, ***, and **** indicate significant differences (p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively) between groups.
8. Spearman’s Correlation Analysis of Physiological and Anti-Inflammatory Effects
In this study, Spearman’s correlation coefficient was used to express the correlation between the two indicators. As shown in Figure 6E, the water content in the stool was negatively correlated with the Shannon index and the concentrations of isobutyric acid, and positively correlated with the levels of IL-6. In addition, the concentration of acetic acid was positively correlated with occludin. The evenness index was negatively correlated with the diarrhea status scores.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9122492
References
- Fernandes, P.; Martens, E. Antibiotics in late clinical development. Biochem. Pharmacol. 2017, 133, 152–163.
- Silverman, M.A.; Konnikova, Y.; Gerber, J.S. Impact of antibiotics on necrotizing enterocolitis and antibiotic-associated diarrhea. Gastroenterol. Clin. N. Am. 2017, 46, 61–76.
- Naghavi, M. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117171.
- Gillespie, D.; Hood, K.; Bayer, A.; Carter, B.; Duncan, D.; Espinasse, A.; Evans, M.; Nuttall, J.; Stanton, H.; Acharjya, A.; et al. Antibiotic prescribing and associated diarrhoea: A prospective cohort study of care home residents. Age Ageing 2015, 44, 853–860.
- Ma, H.; Zhang, L.; Zhang, Y.; Liu, Y.; He, Y.; Guo, L. Combined administration of antibiotics increases the incidence of antibiotic-associated diarrhea in critically ill patients. Infect. Drug Resist. 2019, 12, 1047–1054.
- Hogenauer, C.; Hammer, H.F.; Krejs, G.J.; Reisinger, E.C. Mechanisms and management of antibiotic-associated diarrhea. Clin. Infect. Dis. 1998, 27, 702–710.
- Sammons, J.S.; Toltzis, P.; Zaoutis, T.E. Clostridium difficile infection in children. JAMA Pediatrics 2013, 167, 567–573.
- Szajewska, H.; Mrukowicz, J.Z. Probiotics in prevention of antibiotic-associated diarrhea: Meta-analysis. J. Pediatr. 2003, 142, 85.
- Lee, W.-J.; Hase, K. Gut microbiota–generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424.
- Arpaia, N.; Rudensky, A.Y. Microbial metabolites control gut inflammatory responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2058–2059.
- Lu, Y.-M.; Xie, J.-J.; Peng, C.-G.; Wang, B.-H.; Wang, K.-C.; Li, L.-J. Enhancing clinical efficacy through the gut microbiota: A new field of traditional Chinese medicine. Engineering 2018, 5, 40–49.
- Antunes, L.C.M.; Han, J.; Ferreira, R.; Lolić, P.; Borchers, C.H.; Finlay, B.B. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob. Agents Chemother. 2011, 55, 1494–1503.
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, 1–58.
- Lange, K.W.; Nakamura, Y.; Guo, J.; Chen, N. Diet and medical foods in Parkinson’s disease. Food Sci. Hum. Wellness 2019, 8, 83–95.
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S.
- Zhang, C.; Gong, W.; Li, Z.; Gao, D.; Gao, Y. Research progress of gut flora in improving human wellness. Food Sci. Hum. Wellness 2019, 8, 102–105.
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969.
- Xu, H.-B.; Jiang, R.-H.; Sheng, H.-B. Meta-analysis of the effects of Bifidobacterium preparations for the prevention and treatment of pediatric antibiotic-associated diarrhea in China. Complement. Ther. Med. 2017, 33, 105–113.
- Ling, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Yuan, L.; Li, L.; Xiang, C. Clostridium butyricum combined with bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. BioMed Res. Int. 2015, 2015, 582048.
- Li, Z.; Deng, H.; Zhou, Y.; Tan, Y.; Wang, X.; Han, Y.; Liu, Y.; Wang, Y.; Yang, R.; Bi, Y.; et al. Bioluminescence imaging to track bacteroides fragilis inhibition of vibrio parahaemolyticus infection in mice. Front. Cell. Infect. Microbiol. 2017, 7, 170.
- Hudcovic, T.; Kozáková, H.; Kolínská, J.; Štěpánková, R.; Hrncir, T.; Tlaskalová-Hogenová, H. Monocolonization with Bacteroides ovatus protects immunodeficient SCID mice from mortality in chronic intestinal inflammation caused by long-lasting dextran sodium sulfate treatment. Physiol. Res. 2009, 58, 101–110.
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018, 9, 1040.
- Chng, K.R.; Ghosh, T.; Tan, Y.H.; Nandi, T.; Lee, I.R.; Ng, A.H.Q.; Li, C.; Ravikrishnan, A.; Lim, K.M.; Lye, D.; et al. Metagenome-wide association analysis identifies microbial determinants of post-antibiotic ecological recovery in the gut. Nat. Ecol. Evol. 2020, 4, 1256–1267.
- Masoudi, A.; Raetz, C.R.H.; Zhou, P.; Iv, C.W.P.; Pemble, C.W. Chasing acyl carrier protein through a catalytic cycle of lipid A production. Nature 2013, 505, 422–426.
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772.
This entry is offline, you can click here to edit this entry!
