Loss of elbow motion can lead to disability in everyday gestures, recreational activities, and work. Unfortunately, the elbow joint is particularly prone to stiffness because of its complex anatomy and biomechanics. The etiology of elbow stiffness is varied and must be diagnosed accurately in order to allow optimal treatment, which may be challenging for surgeons and physiotherapists. Its treatment can be either conservative, arthroscopic or surgical, with a trend for arthroscopic procedures when conservative treatment fails. There is no consensus on the optimal imaging workup for elbow joint stiffness, which may have an impact on patient management.
1. Introduction
The elbow joint is a complex hinge-type synovial joint with an important role in the mobilization of the upper limb, linking the hand, wrist, and shoulder. The elbow allows precise hand positioning and serves as a forearm fulcrum maximizing grip strength
[1]. Loss of elbow motion can lead to disability in everyday gestures, recreational activities, or work
[1]. Unfortunately, the elbow joint is particularly prone to stiffness because of its complex anatomy and biomechanics
[2][3]. Post-traumatic changes in the elbow’s peri-articular tissues predispose to capsular calcification and ossification
[4][5][6]. Fractures around the elbow, even if non-displaced and adequately treated, may require sustained immobilization due to difficulties in obtaining a stable osteosynthesis, which may also contribute to joint stiffness
[6]. Histologically, elbow joint stiffness is thought to result from post-traumatic capsular thickening with disorganized collagen fibers, altered cytokine levels, and elevated myofibroblasts
[7].
The etiology of elbow stiffness is varied and must be diagnosed accurately in order to allow the best therapeutic management, which may represent a challenge for surgeons and physiotherapists. Its treatment can be either conservative (e.g., physiotherapy and splinting), arthroscopic (e.g., most frequently: anterior capsular resection, cleansing the humeral fossae, osteophyte and loose bodies ablation) or surgical (e.g., open elbow arthrolysis and prosthetic joint replacement)
[8][9]. Although a well-defined therapeutic algorithm has not yet been proposed, the frequency of postsurgical complications has led to favor arthroscopic procedures when conservative treatment fails
[8][9].
2. Anatomy and Biomechanics
The elbow encompasses three joints within a single articular capsule: humeroradial, humeroulnar, and proximal radioulnar. The distal humerus presents two condyles: the trochlea, medially, which articulates with the greater sigmoid notch of the proximal ulna, and the capitellum, laterally, which articulates with the radial head. The trochlea and the capitellum are anteverted by 30°, and they have a 5° medial rotation and a 6° valgus with respect to the humeral long axis. Anteriorly, radial and coronoid fossae lodge the radial head and coronoid process, respectively, during elbow flexion. Posteriorly, the olecranon fossa lodges the olecranon during extension. The radial head articulates medially with the radial notch of the ulna allowing forearm pronosupination.
The two humeral epicondyles harbor the insertions of various ligaments and tendons. The ulnar collateral ligaments and flexor–pronator tendon group insert onto the more prominent medial epicondyle while the lateral collateral ligament and extensor–supinator tendon group insert onto the less prominent lateral epicondyle
[1].
Stability, mobility, and alignment are essential prerequisites for elbow function
[3]. The maximal elbow flexion–extension range is from 0° to 150° with 75° forearm pronation and an 85° supination
[1]. However, the minimal functional range of motion needed for daily living tasks is 30–130° of flexion–extension with 50° of pronosupination
[10]. Tasks such as using a cell phone or a keyboard require at least 142° of flexion–extension and 65° of pronation
[11]. Elbow stiffness is defined by a flexion–extension range from 30 to 120° or a forearm pronosupination inferior to 45°. Stiffness in flexion is less tolerated than in extension. Loss of supination is more devastating than the loss of pronation, which may be partially compensated by shoulder abduction
[4][12]. Patient activity should also be taken into account, as athletes, musicians, and manual workers may require a greater superior limb range of motion than the general population.
The two main mechanisms that contribute to elbow stiffness are blocks, corresponding to compressive resistance in the direction of the motion, and tethers, corresponding to tensile resistance in the opposite direction of the motion
[13]. Anterior tethers and posterior blocks can cause extension deficit, while posterior tethers and anterior blocks may lead to a flexion deficit (
Table 1). According to Sun et al., tethers can be found alone, but blocks are often associated with tethers
[13].
Table 1. Flexion–extension stiffness etiologies.
3. Clinical Presentation
Acute or repeated trauma remains the most frequent cause of elbow stiffness, followed by osteoarthritis (OA). Elbow pain is usually mechanical in origin and appears in extreme degrees of motion limitation. Spontaneous elbow pain is unusual. In such cases, an infection should be considered and lead to prompt patient management (e.g., blood work, articular puncture/lavage, and antibiotics if septic arthritis is confirmed).
Several classifications of elbow stiffness have been proposed according to the structures involved, anatomic location, mechanism of injury, or severity of motion loss
[2][3]. One of the most relevant classification systems from an imaging and clinical perspective is the one described by Morey et al., which divides elbow pathology into extra-articular; intra-articular, or mixed (the most frequent pattern)
[4]. More recently, Sun et al. proposed a motion-based classification system. Elbow flexion–extension dysfunction is divided into four categories—tethers alone, tethers with blocks, articular malformation, or bony ankyloses, while forearm pronosupination dysfunction is divided into three—contracture alone, radial head malunion/nonunion, or proximal radioulnar bony ankyloses
[13].
3.1. Extra-Articular Elbow Stiffness
Extra-articular elbow stiffness may be related to periarticular tissue pathology (e.g., articular capsule, muscles, ligaments, and skin), heterotopic ossifications, extra-articular bone malalignment or a combination of these. Extra-articular stiffness is frequently posttraumatic, in particular dislocations and complex elbow fractures, although simple nondisplaced radial head fracture or elbow subluxation could lead to stiffness especially after prolonged immobilization. Increased cast immobilization time, alcohol abuse, and prior joint surgery are also considered risk factors
[12].
The diagnosis of skin involvement (usually treated by skin plasty), whether it is a large scar or burn, is clinical and does not necessarily require imaging, unless other associated lesions are suspected, in particular heterotopic ossifications (HO), in the setting of neurogenic paraosteopathy
[13][14]. The latter consists of the formation of mature bone lamellae in soft tissues, which should be differentiated from capsular or ligamentous calcifications or ossifications (
Figure 1). Periarticular HO occurs after direct elbow traumatism (up to 3% in simple dislocation and 20% in fracture–dislocation) and may affect elbow flexion–extension but also lead to a forearm pronosupination deficit due to radioulnar synostosis formation
[15] (
Figure 2). HO risk factors include local burn, concomitant head or spinal injury, prolonged immobilization, and delays before surgery
[16]. There is no consensus on HO treatment, which can be conservative or surgical. Botulinum toxin injections have been shown to be efficient
[17]. Radiation therapy has also been proposed for HO prevention
[16][17]. Joint capsule contractures can be secondary to trauma, arthritis (whether inflammatory, septic, or secondary to repetitive hemarthrosis in hemophilia), and surgery. The anterior capsule seems to be more frequently thickened than the posterior, explaining the preferential loss of extension rather than flexion (
Figure 3). Other soft tissues such as muscle and ligaments can also be involved. Finally, bone malalignment can lead to stiffness especially in cases of extra-articular elbow fracture malunion or congenital anomalies (e.g., congenital dislocation of the radial head or arthrogryposis). Treatment is usually surgical
[13].
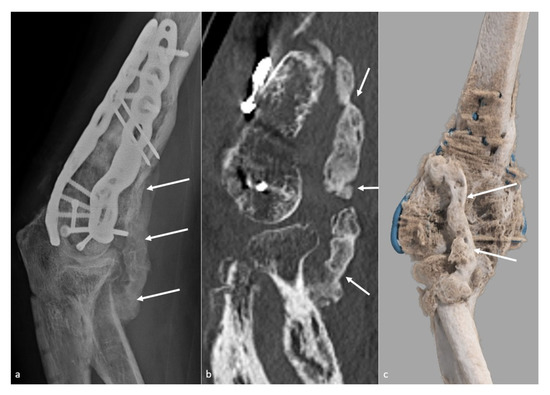
Figure 1. Heterotopic ossification forming a bony bridge between the humerus and the radial neck. Heterotopic ossification (white arrow), leading to severe loss of flexion in a 54-year-old man who suffered from a complex fracture–dislocation, shown (a) on a profile view radiograph, (b) a sagittal CT-scan reformat, and (c) a global illumination 3D reformat.
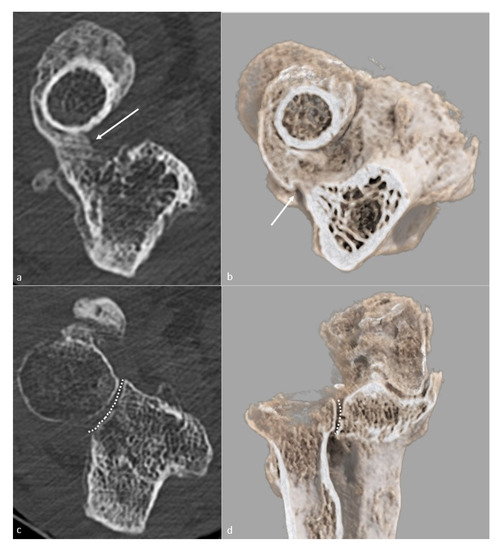
Figure 2. Proximal radioulnar synostosis. Proximal radioulnar synostosis (white arrow), leading to complete loss of forearm rotation in the same patient than in Figure 1, is shown on (a) an axial CT-scan view and (b) a global illumination 3D reformat. Note that the proximal radioulnar joint space (dotted line) can be considered normal and is shown (c) on an axial CT-scan view and (d) a global illumination 3D reformat.
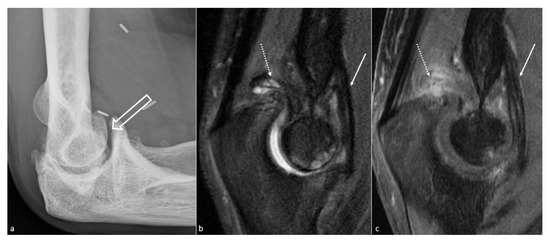
Figure 3. Anterior capsular thickening. (a) Profile view radiograph shows posttraumatic humeroulnar arthrosis (big white arrow), in a 45-year-old woman who suffered from a humeral fracture and presented an extension deficit. (b) Sagittal fat saturated T2-weighted images and (c) fat saturated T1-weighted gadolinium-enhanced images show anterior capsular fibrous thickening (white arrow). Additionally, note the posterior recess synovitis (dotted arrow), with a notable enhancement in (c), whereas no capsular thickening is seen, which also participates in the extension deficit.
3.2. Intra-Articular Stiffness
The mechanisms of Intra-articular stiffness are multiple and can be combined. The most frequent are chondropathy (whether of posttraumatic origin, related to osteochondritis or as part of an OA), primary or secondary synovial chondromatosis, posttraumatic joint surface incongruence, and intra-articular adhesions. Proximal radioulnar joint arthritis, an incongruent radial head (Figure 4), or adhesions between the radial head and the annular ligament may lead to pronosupination deficits.
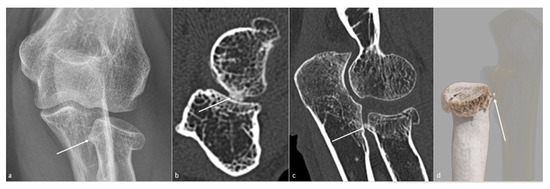
Figure 4. Posttraumatic radial head vicious bone callus. A vicious posttraumatic radial head bone callus (white arrow), causing forearm rotation dysfunction in a 47-year-old woman, is poorly defined on (a) an anteroposterior radiograph. The callus and its particular location are better seen on (b) an axial and (c) sagittal CT-scan reformat, and clearly defined on (d) a global illumination 3D reformat (ulna is voluntarily shown transparent).
OA remains one of the main causes of intra-articular stiffness. It is more often secondary to a traumatic injury, regardless of its severity; however, it can rarely be primitive, especially in manual laborers, weight lifters, and throwing athletes
[18]. Distal humeral fracture and elbow fracture–dislocations are more prone to lead to OA than olecranon or radial head fractures
[19]. In OA, the first mechanism of stiffness is mechanical impingement related to osteophytes at the extremes of flexion and extension (
Figure 5) rather than cartilage surface damage. These osteophytes typically appear in the early stages of osteoarthritis and will classically develop on the tips of the olecranon and the coronoid or fill the coronoid and olecranon fossa (
Figure 6)
[5]. As for extra-articular causes, treatment is usually surgical or arthroscopic
[13]. A total elbow arthroplasty is an option for joint ankyloses or advanced arthropathy
[20].
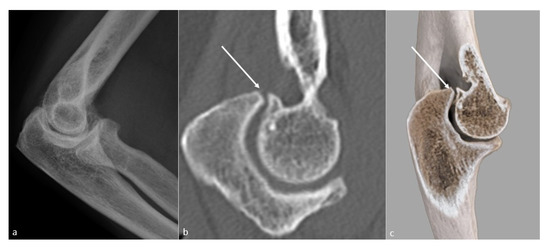
Figure 5. Loss of extension secondary to a bony osteophytic impingement. A bone block secondary to a trochlear osteophyte (white arrow) in a 51-year-old man, manual worker, is shown on (a) a profile view radiograph (not depicted), (b) a sagittal CT-scan reformat, and (c) a global illumination 3D reformat.
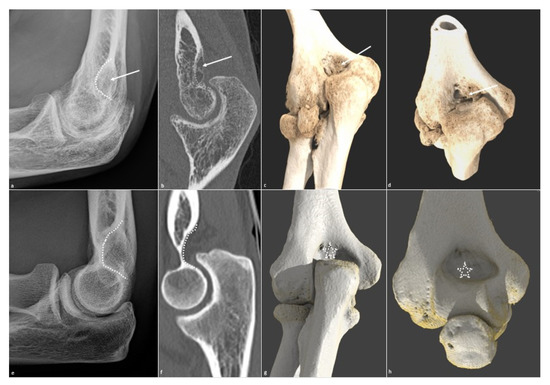
Figure 6. Olecranon fossa osteophytic filling. Osteophytic filling (white arrow) of the olecranon fossa (white dotted line) is shown on (a) a profile radiograph and (b) a sagittal CT-scan in a 26-year-old man who suffered from a humeral fracture and presents extension dysfunction when its corresponding normal aspect is shown in (e,f). The filling is also seen on (c) an extension global illumination 3D reformat and (d) in flexion (white arrows), when the normal aspect is shown in (g,h) (dotted white star).
This entry is adapted from the peer-reviewed paper 10.3390/jcm10225348