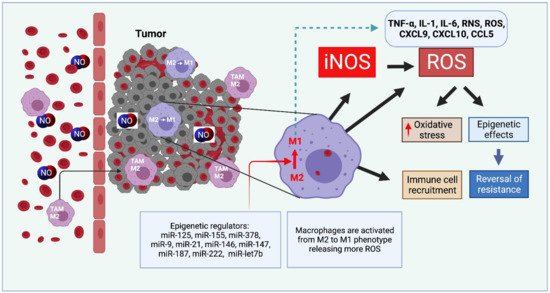
Nitric oxide and its production by iNOS is an established mechanism critical to tumor promotion or suppression. Macrophages have important roles in immunity, development, and progression of cancer and have a controversial role in pro-and anti-tumoral effects. The tumor microenvironment consists of tumor-associated macrophages (TAM), among other cell types that influence the fate of the growing tumor. Depending on the microenvironment and various cues, macrophages polarize into a continuum represented by the M1-like pro-inflammatory phenotype or the anti-inflammatory M2-like phenotype; these two are predominant, while there are subsets and intermediates. Manipulating their plasticity through programming or reprogramming of M2-like to M1-like phenotypes presents the opportunity to maximize tumoricidal defenses. The dual role of iNOS derived NO also influences TAM activity by repolarization to tumoricidal M1-type phenotype. Regulatory pathways and immunomodulation achieve this through miRNA that may inhibit the immunosuppressive tumor microenvironment.
- iNOS/NO
- cancer
- tumor-associated macrophage
- M1-M2
- miRNA
- arginase
- cancer progression
1. Introduction
2. Therapeutic Approaches Utilizing Macrophage-Derived iNOS/NO in Cancer
2.1. iNOS Inhibitors
2.2. NO and Curcumin: A Natural Dietary Compound
2.3. NO and Immunomodulation with microRNAs
2.4. NO-Releasing Nanoparticles
| Nanoparticles and Effect on iNOS or NO | Model System or Cell Type | Effect | Reference |
|---|---|---|---|
| CD44 coated HA-PEI based NPs, miR-125b loaded, iNOS increased | Naïve and KRAS/p53 double mutant nonsmall cell lung cancer (NSCLC) mouse model | Specifically target peritoneal macrophages which reprogram lung TAMs into M1 type | [50] |
| Layered double hydroxides NPs, miR-155 loaded, acidity sensitive, taken up by TAM iNOS increased |
TC-1 mouse tumor model Uptake by TAM Repolarize TAM into M1 |
Synergistic enhancement of therapeutic effects with programmed cell death-1 antibody (α-PD-1) antibody | [40] |
| Lipid-coated calcium phosphonate, miR-155 conjugated mannose, iNOS increased |
S180 mouse sarcoma model | Repolarize M2 into M1 TAMs Significant antitumor effect |
[44] |
| Gold nanoparticles, Photo release of NO |
HeLa | Low doses of Gold nanoparticles were found to produce cytotoxicity as that of 10 g/mL of cisplatin | [49] |
| Poly(D,L-lactic-co-glycolic) acid (PLGA), loaded with ruthenium nitrosyl compounds, NO releasing upon light irradiation |
Melanoma B16-F10 cells | In vitro cytotoxicity assays showed cell death | [51] |
| Cyclodextrin and NO photorelease by a donor |
HeLa, Melanoma, A431- Human squamous carcinoma, Melanoma |
Phototoxicity cell mortality |
[52] [53] [54] |
| Polymeric, NO-releasing |
BE(2)-C, Neuroblastoma cell line | Cisplatin in combination with nanoparticles produced synergistic cytotoxicity | [55] |
| 4-arm branched polymer, NO-releasing | Human head and neck cancer cell line human breast cancer cell lines | Improved cell mortality | [56] |
| Liposome, NO-releasing | Breast cancer cell lines MDA-MB-231 and MDAMB-468 | Improved cell mortality | [57] |
This entry is adapted from the peer-reviewed paper 10.3390/cells10113194
References
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343.
- Kashfi, K. Anti-inflammatory agents as cancer therapeutics. Adv. Pharmacol. 2009, 57, 31–89.
- Kashfi, K. The dichotomous role of H(2)S in cancer cell biology? Déjà vu all over again. Biochem. Pharmacol. 2018, 149, 205–223.
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta 1999, 1411, 401–414.
- Kashfi, K. Nitric oxide in cancer and beyond. Biochem. Pharmacol. 2020, 176, 114006.
- Engels, K.; Knauer, S.; Loibl, S.; Fetz, V.; Harter, P.; Schweitzer, A.; Fisseler-Eckhoff, A.; Kommoss, F.; Hanker, L.; Nekljudova, V.; et al. NO signaling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res. 2008, 68, 5159–5166.
- Bailey, P.; Chang, D.K.; Forget, M.-A.; Lucas, F.A.S.; Alvarez, H.A.; Haymaker, C.; Chattopadhyay, C.; Kim, S.-H.; Ekmekcioglu, S.; Grimm, E.A.; et al. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma. Sci. Rep. 2016, 6, 35848.
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534.
- Nathan, C.; Xie, Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994, 269, 13725–13728.
- Mocellin, S.; Bronte, V.; Nitti, D. Nitric oxide, a double edged sword in cancer biology: Searching for therapeutic opportunities. Med. Res. Rev. 2007, 27, 317–352.
- McGinity, C.L.; Palmieri, E.; Somasundaram, V.; Bhattacharyya, D.; Ridnour, L.; Cheng, R.; Ryan, A.; Glynn, S.; Thomas, D.; Miranda, K.; et al. Nitric Oxide Modulates Metabolic Processes in the Tumor Immune Microenvironment. Int. J. Mol. Sci. 2021, 22, 7068.
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or Not? Int. J. Mol. Sci. 2020, 21, 9393.
- Coulter, J.A.; McCarthy, H.O.; Xiang, J.; Roedl, W.; Wagner, E.; Robson, T.; Hirst, D.G. Nitric oxide—A novel therapeutic for cancer. Nitric Oxide 2008, 19, 192–198.
- De Boo, S.; Kopecka, J.; Brusa, D.; Gazzano, E.; Matera, L.; Ghigo, D.; Bosia, A.; Riganti, C. iNOS activity is necessary for the cytotoxic and immunogenic effects of doxorubicin in human colon cancer cells. Mol. Cancer 2009, 8, 108.
- Lee, M.; Rey, K.; Besler, K.; Wang, C.; Choy, J. Immunobiology of Nitric Oxide and Regulation of Inducible Nitric Oxide Synthase. Results Probl. Cell Differ. 2017, 62, 181–207.
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.; Ramasamay, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94.
- Zheng, X.; Turkowski, K.; Mora, J.; Brüne, B.; Seeger, W.; Weigert, A.; Savai, R. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget 2017, 8, 48436–48452.
- Kudo, S.; Nagasaki, Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J. Control. Release 2015, 217, 256–262.
- Guiducci, C.; Vicari, A.P.; Sangaletti, S.; Trinchieri, G.; Colombo, M.P. Redirecting In vivo Elicited Tumor Infiltrating Macrophages and Dendritic Cells towards Tumor Rejection. Cancer Res. 2005, 65, 3437–3446.
- Sinha, P.; Clements, V.K.; Ostrand-Rosenberg, S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 2005, 174, 636–645.
- Zuo, S.; Song, J.; Zhang, J.; He, Z.; Sun, B.; Sun, J. Nano-immunotherapy for each stage of cancer cellular immunity: Which, why, and what? Theranostics 2021, 11, 7471–7487.
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019, 10, 1205.
- Cheng, R.; Ridnour, L.A.; Glynn, S.A.; Switzer, C.H.; Flores-Santana, W.; Hussain, P.; Thomas, D.D.; Ambs, S.; Harris, C.C.; Wink, D.A. Nitric Oxide and Cancer: An Overview. In Nitric Oxide (NO) and Cancer: Prognosis, Prevention, and Therapy; Bonavida, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–20.
- Saied, E.M.; El-Etreby, N.M. The role and prognostic value of inducible nitric oxide synthase (iNOS) and interleukin-33 (IL-33) in serous and mucinous epithelial ovarian tumours. Ann. Diagn. Pathol. 2017, 27, 62–68.
- De Oliveira, G.A.; Cheng, R.Y.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible Nitric Oxide Synthase in the Carcinogenesis of Gastrointestinal Cancers. Antioxidants Redox Signal. 2017, 26, 1059–1077.
- Switzer, C.H.; Glynn, S.; Ridnour, L.A.; Cheng, R.; Vitek, M.P.; Ambs, S.; Wink, D.A. Nitric oxide and protein phosphatase 2A provide novel therapeutic opportunities in ER-negative breast cancer. Trends Pharmacol. Sci. 2011, 32, 644–651.
- Anttila, M.A.; Voutilainen, K.; Merivalo, S.; Saarikoski, S.; Kosma, V.-M. Prognostic significance of iNOS in epithelial ovarian cancer. Gynecol. Oncol. 2007, 105, 97–103.
- Puhakka, A.; Kinnula, V.; Napankangas, U.; Saily, M.; Koistinen, P.; Paakko, P.; Soini, Y. High expression of nitric oxide synthases is a favorable prognostic sign in non-small cell lung carcinoma. APMIS 2003, 111, 1137–1146.
- Granados-Principal, S.; Liu, Y.; Guevara, M.L.; Blanco, E.; Choi, D.S.; Qian, W.; Patel, T.; A Rodriguez, A.; Cusimano, J.; Weiss, H.L.; et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015, 17, 1–16.
- Heinecke, J.L.; Ridnour, L.A.; Cheng, R.Y.S.; Switzer, C.H.; Lizardo, M.M.; Khanna, C.; Glynn, S.A.; Hussain, S.P.; Young, H.A.; Ambs, S.; et al. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc. Natl. Acad. Sci. USA 2014, 111, 6323–6328.
- Kostourou, V.; Cartwright, J.; Johnstone, A.P.; Boult, J.K.R.; Cullis, E.R.; Whitley, G.; Robinson, S.P. The role of tumour-derived iNOS in tumour progression and angiogenesis. Br. J. Cancer 2010, 104, 83–90.
- Sikora, A.G.; Gelbard, A.; Davies, M.A.; Sano, D.; Ekmekcioglu, S.; Kwon, J.; Hailemichael, Y.; Jayaraman, P.; Myers, J.N.; Grimm, E.A.; et al. Targeted Inhibition of Inducible Nitric Oxide Synthase Inhibits Growth of Human Melanoma In vivo and Synergizes with Chemotherapy. Clin. Cancer Res. 2010, 16, 1834–1844.
- Lu, G.; Zhang, R.; Geng, S.; Peng, L.; Jayaraman, P.; Chen, C.; Xu, F.; Yang, J.; Li, Q.; Zheng, H.; et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 2015, 6, 6676.
- Piao, L.; Mukherjee, S.; Chang, Q.; Xie, X.; Li, H.; Castellanos, M.R.; Banerjee, P.; Iqbal, H.; Ivancic, R.; Wang, X.; et al. TriCurin, a novel formulation of curcumin, epicatechin gallate, and resveratrol, inhibits the tumorigenicity of human papillomavirus-positive head and neck squamous cell carcinoma. Oncotarget 2016, 8, 60025–60035.
- Zhang, X.; Tian, W.; Cai, X.; Wang, X.; Dang, W.; Tang, H.; Cao, H.; Wang, L.; Chen, T. Hydrazinocurcumin Encapsuled Nanoparticles “Re-Educate” Tumor-Associated Macrophages and Exhibit Anti-Tumor Effects on Breast Cancer Following STAT3 Suppression. PLoS ONE 2013, 8, e65896.
- Mukherjee, S.; Hussaini, R.; White, R.; Atwi, D.; Fried, A.; Sampat, S.; Piao, L.; Pan, Q.; Banerjee, P. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV+ tumors. Cancer Immunol. Immunother. 2018, 67, 761–774.
- Mukherjee, S.; Baidoo, J.; Fried, A.; Atwi, D.; Dolai, S.; Boockvar, J.; Symons, M.; Ruggieri, R.; Raja, K.; Banerjee, P. Curcumin changes the polarity of tumor-associated microglia and eliminates glioblastoma. Int. J. Cancer 2016, 139, 2838–2849.
- Squadrito, M.L.; Etzrodt, M.; De Palma, M.; Pittet, M.J. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013, 34, 350–359.
- Lin, Y.-X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics 2020, 10, 281–299.
- Yang, L.; Sun, J.; Liu, Q.; Zhu, R.; Yang, Q.; Hua, J.; Zheng, L.; Li, K.; Wang, S.; Li, A. Synergetic Functional Nanocomposites Enhance Immunotherapy in Solid Tumors by Remodeling the Immunoenvironment. Adv. Sci. 2019, 6, 1802012.
- Parayath, N.N.; Gandham, S.K.; Leslie, F.; Amiji, M.M. Improved anti-tumor efficacy of paclitaxel in combination with MicroRNA-125b-based tumor-associated macrophage repolarization in epithelial ovarian cancer. Cancer Lett. 2019, 461, 1–9.
- Reichel, D.; Tripathi, M.; Perez, J.M. Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment. Nanotheranostics 2019, 3, 66–88.
- Scodeller, P.; Gracia, L.S.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Säälik, P.; Kurm, K.; Squadrito, M.L.; Kotamraju, V.R.; Rinken, A.; et al. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci. Rep. 2017, 7, 1–12.
- Zang, X.; Zhang, X.; Zhao, X.; Hu, H.; Qiao, M.; Deng, Y.; Chen, D. Targeted Delivery of miRNA 155 to Tumor Associated Macrophages for Tumor Immunotherapy. Mol. Pharm. 2019, 16, 1714–1722.
- Seabra, A.B.; de Lima, R.; Calderón, M. Nitric oxide releasing nanomaterials for cancer treatment: Current status and perspectives. Curr. Top. Med. Chem. 2015, 15, 298–308.
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2020, 6, 1973–1987.
- Kandoth, N.; Vittorino, E.; Sciortino, M.T.; Parisi, T.; Colao, I.; Mazzaglia, A.; Sortino, S. A Cyclodextrin-Based Nanoassembly with Bimodal Photodynamic Action. Chem. - A Eur. J. 2011, 18, 1684–1690.
- Stevens, E.V.; Carpenter, A.W.; Shin, J.H.; Liu, J.; Der, C.J.; Schoenfisch, M.H. Nitric Oxide-Releasing Silica Nanoparticle Inhibition of Ovarian Cancer Cell Growth. Mol. Pharm. 2010, 7, 775–785.
- Sudhesh, P.; Tamilarasan, K.; Arumugam, P.; Berchmans, S. Nitric Oxide Releasing Photoresponsive Nanohybrids As Excellent Therapeutic Agent for Cervical Cancer Cell Lines. ACS Appl. Mater. Interfaces 2013, 5, 8263–8266.
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579.
- Gomes, A.J.; Espreafico, E.M.; Tfouni, E. trans-(PF6)2 and incorporated in PLGA nanoparticles for the delivery of nitric oxide to B16-F10 cells: Cytotoxicity and phototoxicity. Mol. Pharm. 2013, 10, 3544–3554.
- Deniz, E.; Kandoth, N.; Fraix, A.; Cardile, V.; Graziano, A.C.E.; Furno, D.L.; Gref, R.; Raymo, F.M.; Sortino, S. Photoinduced Fluorescence Activation and Nitric Oxide Release with Biocompatible Polymer Nanoparticles. Chem.-A Eur. J. 2012, 18, 15782–15787.
- Kandoth, N.; Kirejev, V.; Monti, S.; Gref, R.; Ericson, M.; Sortino, S. Two-Photon Fluorescence Imaging and Bimodal Phototherapy of Epidermal Cancer Cells with Biocompatible Self-Assembled Polymer Nanoparticles. Biomacromolecules 2014, 15, 1768–1776.
- Fraix, A.; Kandoth, N.; Manet, I.; Cardile, V.; Graziano, A.C.E.; Gref, R.; Sortino, S. An engineered nanoplatform for bimodal anticancer phototherapy with dual-color fluorescence detection of sensitizers. Chem. Commun. 2013, 49, 4459–4461.
- Duong, H.T.T.; Kamarudin, Z.M.; Erlich, R.B.; Li, Y.; Jones, M.W.; Kavallaris, M.; Boyer, C.; Davis, T.P. Intracellular nitric oxide delivery from stable NO-polymeric nanoparticle carriers. Chem. Commun. 2012, 49, 4190–4192.
- Duan, S.; Cai, S.; Yang, Q.; Forrest, M.L. Multi-arm polymeric nanocarrier as a nitric oxide delivery platform for chemotherapy of head and neck squamous cell carcinoma. Biomaterials 2012, 33, 3243–3253.
- Lee, S.Y.; Rim, Y.; McPherson, D.D.; Huang, S.-L.; Kim, H. A novel liposomal nanomedicine for nitric oxide delivery and breast cancer treatment. Bio-Med. Mater. Eng. 2014, 24, 61–67.
