Sugammadex (Bridion®, Merk Sharp and Dohme Corp., Kenilworth, NJ, USA) is a modified cyclodextrin designed to selectively encapsulate aminosteroidal neuromuscular blocking agents (NMBAs) such as rocuronium and vecuronium, which leads to the rapid reversal of neuromuscular block (NMB).
- kidney failure
- chronic
- renal insufficiency
- chronic
- rocuronium
1. Introduction
Sugammadex administered to the blood rapidly encapsulates the NMBA, leading to an increased gradient in the concentration of NMBA between the neuromuscular junction and plasma; subsequently, the NMBA present at the neuromuscular junction is rapidly released into the blood, and rapid NMB reversal is achieved [1]. The sugammadex-NMBA complex produced is inactive and hydrophilic and is mainly excreted by the kidney. In addition, NMBAs such as rocuronium or vecuronium are excreted mainly through the kidneys [2][3]. In patients with severe renal impairment, the pharmacokinetics of both rocuronium and sugammadex are altered, and, thus, the NMB reversal by sugammadex can be unpredictable or incomplete [2]. Therefore, the U.S. Food and Drug Administration does not recommend sugammadex for patients with a creatinine clearance of <30 mL min −1 [4].
Nevertheless, the use of sugammadex is often observed in clinical practice for surgical patients with chronic kidney disease in various clinical situations, and some prospective case-control or retrospective studies and case reports regarding the use of sugammadex in patients with end-stage renal disease (ESRD) have been published [5][6][7]. To date, no systematic review regarding the use of sugammadex in patients with severe renal impairment has been reported, while there have been several meta-analyses showing the effectiveness, safety, and superiority of sugammadex, compared to cholinesterase inhibitors for NMB reversal in adult patients without organ dysfunction; a systematic review would need to take into account the results of the studies that reported the use of sugammadex in patients with ESRD and analyze their pooled data.
2. Study Characteristics and Patient Demographics
| Study | Journal | Study Design | Center/Country | Group R (n) | Group N (n) | Age | Sugammadex Dose | NMB Monitoring |
|---|---|---|---|---|---|---|---|---|
| de Souza et al., 2015 [8] | European Journal of Anaesthe-siology | Prospective clinical trial | Two hospitals Brazil, and Spain | ClCr < 30 mL min−1 (20) |
ClCr > 90 mL min−1 (20) |
18–65 | 4 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Panhuizen et al., 2015 [9] | British Journal of Anaesthesia | Case control comparative study | Eight centers in Europe | ClCr < 30 mL min−1 (35) |
ClCr ≥ 80 mL min−1 (35) |
≥18 | 4 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Staals et al., 2008 [10] |
British Journal of Anaesthesia | Prospective clinical trial | Three centers in Europe | ClCr < 30 mL min−1 (15) |
ClCr ≥ 80 mL min−1 (15) |
≥18 | 2 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Staals et al., 2010 [2] |
British Journal of Anaesthesia | Prospective clinical trial | Three centers in Europe | ClCr < 30 mL min−1 (15) |
ClCr ≥ 80 mL min−1 (15) |
≥18 | 2 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Maeyama et al., 2014 [11] | European Journal of Anaesthesiology | Prospective clinical trial | University hospital, Japan | ClCr < 15mL min−1 (13) |
ClCr > 90 mL min−1 (14) |
≥18 | 4 mg kg−1 | Not mentioned |
| Min et al., 2017 [12] |
International Journal of Clinical Pharmacology and Therapeutics | Open label, two parts, phase 1 study | Clinical pharma-cology of Miami, USA | ClCr < 30 mL min−1 (6) |
ClCr ≥ 80 mL min−1 (6) |
≥18 | 4 mg kg−1 | None |
| Study ID | Journal | Study Design | Center/Country | Enrolled Criteria (n) | Age | NMB Reversal Agent |
|---|---|---|---|---|---|---|
| Adams et al., 2020 [6] | Anaesthesia | Two centers retrospective study | Pittsburgh Medical Center, Memorial Sloan Kettering Cancer Center, USA | End-stage renal disease which is mandatory for renal replacement therapy (158) | ≥18 | sugammadex |
| Paredes et al., 2020 [13] | Canadian Journal of Anaesthesia | Historical cohort study, three-distinct geographic locations | Scottsdale, AZ, Jacksonville, FL, Rochester, MN, USA | eGFR value < 15 mL min−1 (219) | ≥18 | sugammadex |
| Ono et al., 2018 [5] | Journal of Anesthesia Clinical Reports | Retrospective study | Aichi Medical University, Nagakute, Japan | Diagnosed severe with renal failure and underwent renal transplantation (99) | not mentioned | sugammadex |
3. Main Results: The Primary and Secondary Outcomes of the Included Prospective Studies
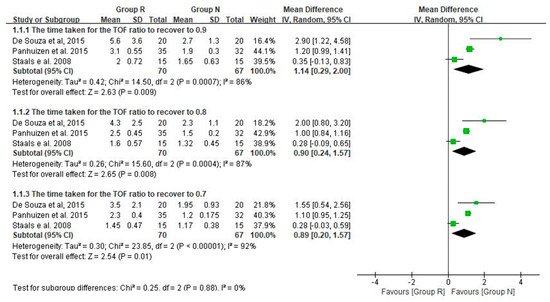
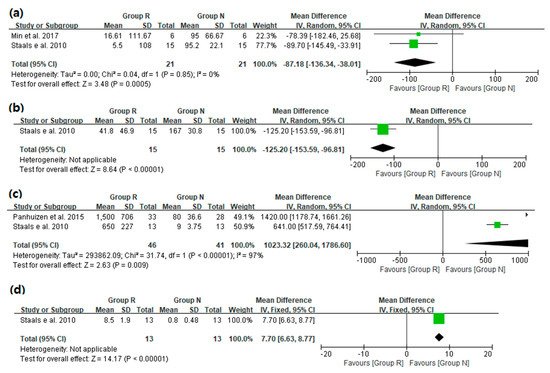
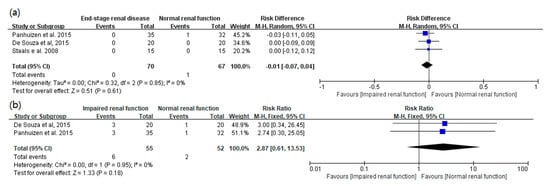
4. The Results of the Included Retrospective Studies
5. Risk of Bias in the Prospective Case-Control Studies
| Study | Confounding | Selection | Classification of Interventions | Deviation from Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | ROBINS-I Overall |
|---|---|---|---|---|---|---|---|---|
| de Souza et al., 2015 [8] | Moderate | Moderate | Moderate | Moderate | Moderate | Serious | Low | Serious |
| Panhuizen et al., 2015 [9] | Moderate | Low | Moderate | Low | Moderate | Serious | Low | Serious |
| Staals et al., 2008 [10] |
Moderate | Moderate | Low | Low | Low | Serious | Low | Serious |
| Staals et al., 2010 [2] |
Moderate | Moderate | Low | Low | Moderate | Serious | Low | Serious |
| Maeyama et al., 2014 [11] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Min et al., 2017 [12] |
Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
5. Discussion
This entry is adapted from the peer-reviewed paper 10.3390/medicina57111259
References
- Epemolu, O.; Bom, A.; Hope, F.; Mason, R. Reversal of neuromuscular blockade and simultaneous increase in plasma rocuronium concentration after the intravenous infusion of the novel reversal agent Org 25969. Anesthesiology 2003, 99, 632–637.
- Staals, L.M.; Snoeck, M.M.; Driessen, J.J.; van Hamersvelt, H.W.; Flockton, E.A.; van den Heuvel, M.W.; Hunter, J.M. Reduced clearance of rocuronium and sugammadex in patients with severe to end-stage renal failure: A pharmacokinetic study. Br. J. Anaesth. 2010, 104, 31–39.
- Peeters, P.; Passier, P.; Smeets, J.; Zwiers, A.; de Zwart, M.; van de Wetering-Krebbers, S.; van Iersel, M.; van Marle, S.; van den Dobbelsteen, D. Sugammadex is cleared rapidly and primarily unchanged via renal excretion. Biopharm. Drug Dispos. 2011, 32, 159–167.
- U.S. Food and Drug Administration; Merck Sharp & Dohme Corp. BRIDION® (Sugammadex) Injection, for Intravenous Use; Initial U.S. Approval: Silver Spring, MD, USA, 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022225lbl.pdf (accessed on 5 December 2019).
- Ono, Y.; Fujita, Y.; Kajiura, T.; Okawa, H.; Nakashima, J.; Isobe, H.; Fujiwara, Y. Efficacy and safety of sugammadex in patients undergoing renal transplantation. JA Clin. Rep. 2018, 4, 56.
- Adams, D.R.; Tollinche, L.E.; Yeoh, C.B.; Artman, J.; Mehta, M.; Phillips, D.; Fischer, G.W.; Quinlan, J.J.; Sakai, T. Short-term safety and effectiveness of sugammadex for surgical patients with end-stage renal disease: A two-centre retrospective study. Anaesthesia 2020, 75, 348–352.
- Pfaff, K.; Tumin, D.; Tobias, J.D. Sugammadex for Reversal of Neuromuscular Blockade in a Patient With Renal Failure. J. Pediatr. Pharmacol. Ther. 2019, 24, 238–241.
- De Souza, C.M.; Tardelli, M.A.; Tedesco, H.; Garcia, N.N.; Caparros, M.P.; Alvarez-Gomez, J.A.; de Oliveira, I.S., Jr. Efficacy and safety of sugammadex in the reversal of deep neuromuscular blockade induced by rocuronium in patients with end-stage renal disease: A comparative prospective clinical trial. Eur. J. Anaesthesiol. 2015, 32, 681–686.
- Panhuizen, I.F.; Gold, S.J.; Buerkle, C.; Snoeck, M.M.; Harper, N.J.; Kaspers, M.J.; van den Heuvel, M.W.; Hollmann, M.W. Efficacy, safety and pharmacokinetics of sugammadex 4 mg kg-1 for reversal of deep neuromuscular blockade in patients with severe renal impairment. Br. J. Anaesth. 2015, 114, 777–784.
- Staals, L.M.; Snoeck, M.M.; Driessen, J.J.; Flockton, E.A.; Heeringa, M.; Hunter, J.M. Multicentre, parallel-group, comparative trial evaluating the efficacy and safety of sugammadex in patients with end-stage renal failure or normal renal function. Br. J. Anaesth. 2008, 101, 492–497.
- Maeyama, A.; Nagasaka, H.; Matsumoto, N. Prolonged recovery time from muscle relaxation due to rocuronium in patients with severe chronic renal disease using sugammadex: 9AP4-6. Eur. J. Anaesthesiol. EJA 2014, 31, 152.
- Min, K.C.; Lasseter, K.C.; Marbury, T.C.; Wrishko, R.E.; Hanley, W.D.; Wolford, D.G.; Udo de Haes, J.; Reitmann, C.; Gutstein, D.E. Pharmacokinetics of sugammadex in subjects with moderate and severe renal impairment. Int. J. Clin. Pharmacol. Ther. 2017, 55, 746–752.
- Paredes, S.; Porter, S.B.; Porter, I.E., 2nd; Renew, J.R. Sugammadex use in patients with end-stage renal disease: A historical cohort study. Can. J. Anaesth. 2020, 67, 1789–1797.
- Magoon, R.; Kashav, R.; Kohli, J.K. Sugammadex in end-stage renal disease: Too early for a “free-pass”. Can. J. Anaesth. 2021, 68, 264–265.
- Carlos, R.V.; Torres, M.L.; de Boer, H.D. The use of rocuronium and sugammadex in paediatric renal transplantation: Two case reports. Eur. J. Anaesthesiol. 2016, 33, 383–386.
- Kurita, S.; Moriwaki, K.; Shiroyama, K.; Sanuki, M.; Toyota, Y.; Takebayashi, M. Rocuronium-sugammadex use for electroconvulsive therapy in a hemodialysis patient: A case report. JA Clin. Rep. 2016, 2, 28.
- Renew, J.R.; Porter, S.B.; Porter, I.; Paredes, S. In reply: Sugammadex in end-stage renal disease: Too early for a “free-pass”. Can. J. Anaesth. 2021, 68, 266–267.
