Cellular communication plays a critical role in diverse aspects of tumorigenesis including tumor cell growth/death, adhesion/detachment, migration/invasion, angiogenesis, and metastasis. G protein-coupled receptors (GPCRs) which constitute the largest group of cell surface receptors are known to play fundamental roles in all these processes. When considering the importance of GPCRs in tumorigenesis, the adhesion GPCRs (aGPCRs) are unique due to their hybrid structural organization of a long extracellular cell-adhesive domain and a seven-transmembrane signaling domain. Indeed, aGPCRs have been increasingly shown to be associated with tumor development by participating in tumor cell interaction and signaling. ADGRG1/GPR56, a representative tumor-associated aGPCR, is recognized as a potential biomarker/prognostic factor of specific cancer types with both tumor-suppressive and tumor-promoting functions.
1. Introduction
G protein-coupled receptors (GPCRs) play a central role in cellular communication [
1,
2,
3]. Aberrant expression and/or genetic mutations of GPCRs are often identified in various cancer types, manifesting either tumor-promoting or tumor-suppressive functions [
4,
5,
6]. Furthermore, GPCR transactivation of different signaling molecules such as the receptor tyrosine kinases (RTKs) are closely linked to tumorigenesis [
7,
8]. Therefore, GPCRs and their cognate ligands as well as the specific signaling networks induced by the GPCR-ligand interaction are the key areas of interest for cancer research.
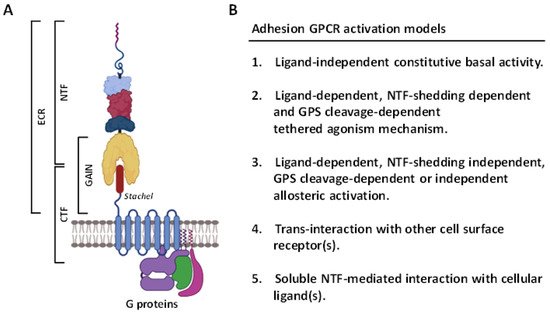
In recent years, the GPCR superfamily has been further classified based upon their phylogenetic characteristics and subdivided into five main families, including glutamate, rhodopsin, adhesion, frizzled/Taste2, and secretin (GRAFS) [
9]. Among these, the adhesion GPCRs (aGPCRs) have several unusual structural and functional features [
10] (
Figure 1). Firstly, the extracellular region (ECR) of most aGPCRs is uncommonly large and often consists of diverse cell-adhesive protein modules such as the epidermal growth factor (EGF)-like, immunoglobulin (Ig)-like, and lectin-like domains at its N-terminal half. These protein modules normally function as the binding sites for specific cellular ligands and/or binding partners of aGPCRs [
10,
11]. Secondly, the majority of aGPCRs comprise a signature GPCR autoproteolysis-inducing (GAIN) domain at the C-terminal half of ECR. A self-catalytic proteolytic reaction normally occurs during receptor biosynthesis at the consensus GPCR proteolysis site (GPS) within the GAIN domain [
12,
13,
14]. As a result, GPS cleavage disjoints the receptor into a non-covalent dual-subunit complex comprising an N-terminal extracellular fragment (NTF) and a C-terminal 7TM fragment (CTF) [
12,
13,
14] (
Figure 1A). Thirdly, the activation and signaling mechanisms of aGPCRs are multifaceted, including the GPS cleavage-dependent and -independent as well as NTF-CTF dissociation-dependent and -independent modes [
10,
15,
16,
17]. Nevertheless, a tethered agonism model is well accepted as the common activation mechanism for most aGPCRs [
18,
19]. This involves the unmasking and binding of a tethered agonistic
Stachel peptide with its own 7TM, following the separation of NTF from CTF caused by the binding of its cellular ligand(s) to the ECR [
20] (
Figure 1B).
Figure 1. Structural features and activation mechanisms of adhesion GPCRs. (A) Schematic depicting the structural organization of aGPCRs in general. The ECR contains multiple cell-adhesive protein domains (indicated by different colored lines and shapes) followed by a GAIN domain. The Stachel peptide is depicted as a brown cylinder. ECR, extracellular region; CTF, C-terminal fragment; NTF, N-terminal fragment; GAIN, GPCR autoproteolysis-inducing. Illustration was generated using the Biorender software. (B) The diverse potential activation mechanisms mediated by aGPCRs.
2. Overview of the ADGRG1/GPR56 Receptor
2.1. Structural Characteristics of the ADGRG1/GPR56 Protein
The full-length human ADGRG1/GPR56 receptor is a protein of 693 amino acids encoded by the
ADGRG1 gene on the chromosome 16q21 [
24]. However, extensive RNA splicing of its transcripts is predicted to generate at least five different GPR56 protein isoforms that are different in the composition of ECR and/or the first intracellular loop. These GPR56 receptor variants have been found to have differential functional and signaling characteristics and are discussed later [
25,
26]. Moreover, the usage of multiple alternative transcription start sites has been identified to generate many more diverse GPR56 transcript variants; however, the frequencies of these transcripts and the resulting protein isoforms remain to be fully investigated [
27,
28].
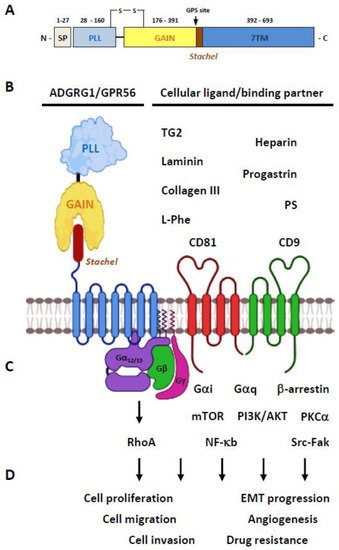
The GPR56 protein consists of a N-terminal PLL (pentraxin/laminin/neurexin sex-hormone-binding globulin-like) domain and a C-terminal GAIN domain in its ECR (
Figure 2A) [
26]. Of interest, the PLL domain shares only a relatively weak homology with either the pentraxin or laminin/neurexin/sex-hormone-binding globulin domain hence is a rather novel structural module unique to GPR56 [
26]. The GPR56-GAIN domain is unusually small in comparison to those of other aGPCRs. Indeed, its GAIN sub-domain A contains only three α-helices compared to the six α-helices identified in the canonical GAIN sub-domain A of ADGRL1/Latrophilin-1 and ADGRB3/BAI-3 [
13,
26,
29]. Nevertheless, the GAIN sub-domain B of GPR56 seems to be comparable with those of other aGPCRs by having 13 β-strands and 2 small α-helices. The proteolytic cleavage of GPR56 takes place between Leu
382 and Thr
383 at the consensus GPS motif located between the 12th and 13th β-strands of the GAIN sub-domain B [
26]. Interestingly, the PLL domain is absent in two alternatively-spliced GPR56 isoforms, while the other three GPR56 variants contain essentially the same full-length ECR [
25]. Altogether, GPR56 is a fully-processed archetypal aGPCR consisting of non-covalently associated NTF-CTF receptor subunits (
Figure 2B).
Figure 2. Overview of the ADGRG1/GPR56 protein. (A) Schematic depicting the domain organization of the GPR56 receptor protein. The light-purple and light-yellow rectangles represent the PLL and GAIN domains, respectively. The 7TM region is represented by a light blue rectangle. The Stachel tethered peptide is indicated by a brown rectangle. SP, signal peptide; GPS, GPCR proteolysis site. (B) Diagrams showing the GPR56 receptor and its known cellular ligands and binding partners. (C) The GPR56-mediated signaling pathways reported to date. (D) The tumorigenic functions known to be mediated by GPR56. Illustration was generated using the Biorender software.
2.2. Ligands/Binding Partners of the ADGRG1/GPR56 Protein
In terms of specific cellular ligands/binding partners, GPR56 was one of the first aGPCRs to be deorphanized (
Figure 2B). Little et al. showed that the CD9 and CD81 tetraspanins form a complex specifically with GPR56 and many G protein subunits, including Gα
q, Gα
11, and Gβ [
30]. This GPR56-tetraspanin-G protein complex was important for subsequent signaling regulation, hence suggesting a regulatory role for CD9 and CD81 as the membrane scaffolding proteins of GPR56. Xu and colleagues identified tissue transglutaminase (also named TG2) as the specific extracellular matrix (ECM) ligand of melanoma cells [
31]. Critically, it was found that the TG2-GPR56 interaction inhibited the growth and metastasis of melanoma tumors [
32,
33,
34]. Piao’s group subsequently discovered another ECM ligand of GPR56, the type III collagen (collagen-III), in the mouse brain [
35]. The interaction of collagen-III and GPR56 activated the Gα
12/13-RhoA signaling pathway and played an essential role in the proper lamination of the cerebral cortex in the developing brain. Later studies by the same group also identified a different ECM ligand, laminin, for GPR56 [
36]. It was revealed that the GPR56 receptor in oligodendrocyte precursor cells (OPCs) responded to the microglia-derived TG2 in the presence of laminin. Recently, the PLL domain was identified as the specific binding site for collagen-III and TG2 on GPR56 [
37,
38].
As well as protein ligands, heparin was recently identified by Chiang et al. as a novel glycosaminoglycan (GAG) binding partner of GPR56 [
39]. Interestingly, heparin-GPR56 interaction reduced the shedding of its NTF and enhanced cell adhesion and motility [
39]. Jin et al. demonstrated that progastrin, an 80 aa-long precursor of the peptide hormone gastrin, bound directly to GPR56 expressed in colonic stem/progenitor cells and colon cancer cells to promote cellular proliferation [
40]. More recently, Chen and colleagues screened a large number of bioactive metabolites from intestinal microbiota and identified the essential amino acid L-Phe, found abundantly in the culture supernatants of the species
B. theta (
B. theta C34), as a small molecule agonist of GPR56 and ADGRG3/GPR97 [
41]. Functional analyses showed that the ECR of GPR56 was required for the receptor activation induced by L-Phe. Nevertheless, GPR56 activation by L-Phe required very high concentrations of L-Phe (>1 mM); hence, the physiological significance of this ligand-receptor interaction remained unanswered. Lastly, Li et al. identified phosphatidylserine (PS) as an isoform-specific ligand of the GPR56-S4 variant restrictedly expressed in microglial cells [
42]. The GPR56-S4 isoform lacked the entire PLL domain, but the specific PS-S4 isoform interaction was shown to be critical for microglia-mediated synaptic pruning during brain development [
42].
Finally, receptor-specific monoclonal antibodies (mAbs), polyclonal antibodies (pAbs), and monobodies have been generated to serve as the artificial ligands of GPR56 for various functional studies [
43,
44,
45,
46,
47]. Moreover, 3-α-acetoxydihydrodeoxygedunin (3-α-DOG) and dihydromunduletone (DHM) were identified as a selective small chemical agonist and antagonist of GPR56, respectively [
48,
49,
50].
2.3. Activation and Signaling Mechanisms of ADGRG1/GPR56
Diverse activation mechanisms of the GPR56 receptor, including the GAIN domain-dependent/-independent and GPS cleavage-dependent/-independent modes, have been identified (
Figure 2C) [
18,
47,
51,
52]. However, increasing evidence suggests an unusual signaling transduction mechanism in which the GPR56-NTF acts as a repressor of the basal GPR56 signaling. Indeed, NTF-truncated GPR56 receptors are constitutively active displaying increased SRF and NFAT activities, TGF-α shedding, β-arrestin binding, and receptor ubiquitination when expressed in transfected cells [
51,
52]. Likewise, deletion of the PLL domain increases the signaling activity of GPR56 [
26]. These results strongly suggest that the activation of GPR56 (and likely most other aGPCRs) is mostly mediated via the dissociation/conformational change of the NTF. This permits a newly exposed tethered-agonist peptide within the CTF known as the
Stachel sequence to self-activate receptor signaling [
18,
19].
As such, in comparison to the wild-type (WT) GPR56-CTF, CTF variants of GPR56 with truncated N-terminal regions showed reduced abilities to activate the Gα
13 protein and the serum response element (SRE)-luciferase activity in transfected cells. On the other hand, synthetic peptides of the β-strand 13 of GPR56 GAIN sub-domain B were sufficient to induce the signaling activity of GPR56 [
18]. Therefore, it was established that following the dissociation of NTF, the
Stachel peptide of GPR56-CTF was exposed to interact with its own 7TM moiety, leading to conformational changes and activation of the receptor. Most recent studies have further substantiated this novel activation mechanism by demonstrating that binding of cognate ligands such as collagen-III and activating mAbs elicit GPR56 activation and Gα
12/13-RhoA signaling after the ligand-induced NTF-CTF dissociation [
44,
48,
53].
Recently, it was shown that the GPR56 receptor still remains constitutively active after the removal of its entire ECR. Nevertheless, this ECR-less GPR56 receptor variant only activated NFAT and induced TGF-α shedding, but not the SRF activity. This finding thus suggested a GAIN domain-independent mechanism of GPR56 activation [
51,
54]. On the other hand, 3-α-DOG was able to activate the GPS cleavage-deficient GPR56-F385A mutant [
48,
50]. Likewise, several GPR56-specific monobodies were found to activate the same GPR56-F385A mutant efficiently [
47]. These results indicated that these specific agonists bind to the ECR of GPR56 and cause a unique conformational change that activates the receptor without the need of its proteolytic modification, hence supporting the GPS cleavage-independent activation mechanism of GPR56 [
55].
Similar to the various GPR56 activation mechanisms described above, diverse GPR56-mediated signaling pathways were reported. Notably, most of the GPR56 signaling studies have shown the specific involvement of the Gα
12/13-RhoA signaling axis as mentioned previously [
56]. Nevertheless, additional signaling pathways involving Gα
i, Gα
q, Gβγ, β-arrestin, mTOR, PKCα, NF-κb, and Src-Fak have also been described elsewhere (
Figure 2C) [
30,
32,
52,
57,
58,
59]. In conclusion, multiple activation and signaling pathways of GPR56 have been identified that seem to depend mainly on the specific ligands and cell types studied.
2.4. The Biological Functions of GPR56
Due to its broad mRNA expression patterns in the brain, kidney, testis, thyroid, pancreas, skeletal muscle and the hematopoietic system (BioGPS.org), GPR56 is a functionally versatile aGPCR involved in many physiological processes. In brief, the highest GPR56 expression level was detected in CD56
+ NK cells, although cytotoxic CD8
+, CD4
+, and γδ T lymphocytes also expressed significant levels of GPR56 [
60,
61]. In the nervous systems, GPR56 expression was detected in Cajal–Retzius cells, radial glial cells, Tuj1
+ migrating neurons, OPCs, Schwann cells (SCs), and microglial cells [
35,
36,
62,
63,
64,
65]. GPR56 was identified as the most abundantly expressed GPCR in the pancreatic islets, especially β-cells [
66]. GPR56 was expressed in mouse Sertoli cells as well as Mullerian duct epithelium of avian female gonads [
67,
68]. For the general review of the role of GPR56 in health and disease, the readers are referred to a recent review article [
69].
As the single disease gene responsible for bilateral frontoparietal polymicrogyria (BFPP), GPR56 is undoubtedly best known for its essential role in normal cerebral cortical development [
24,
35]. Subsequent studies showed that GPR56 is also critically involved in the neuronal myelination of the CNS and peripheral nervous system (PNS), myelin repair in CNS neurons, the proliferation of OPCs, proper radial axonal sorting by SCs, microglia-mediated synaptic pruning, and antidepressant response [
36,
62,
63,
64,
70,
71].
In addition to the nervous systems, GPR56 has been shown to play a regulatory role in myoblast fusion and mechanical overload-induced muscle hypertrophy, NK cell cytotoxic activity, hemostatic shear-force sensing by platelets, pancreatic islet function, the development and differentiation of hematopoietic stem/progenitor cell, male gonad development in mice, as well as the normal development of avian embryonic Müllerian duct [
43,
59,
66,
67,
68,
72,
73,
74,
75,
76,
77,
78,
79].
Importantly, GPR56 was often detected and implicated in the development of many different types of cancers, including melanoma, breast, non-small cell lung, esophageal squamous cell cancer [
80], ovarian, colon, pancreatic carcinoma [
81,
82], glioblastoma/astrocytoma [
83], and leukemia [
79,
84,
85]. In general, GPR56 was shown to regulate cell growth, adhesion, and migration of diverse cancer cell types. In addition, a role for GPR56 in modulating the epithelial-mesenchymal transition (EMT), angiogenesis, and chemo-/radioresistance of cancer cells has been reported (
Figure 2D). Overall, increasing evidence has indicated that GPR56 might function as the biomarker/prognostic factor of certain cancers and a potential tumor-promoter or tumor-suppressor for others.
3. ADGRG1/GPR56 as a Cancer Marker and/or Prognostic Factor
Due to its restricted expression in certain stages of tumor development or the association of its expression levels with the metastatic stage or survival rate of cancer patients, GPR56 was thought to act as a potential biomarker and/or prognostic factor of certain cancers. Identified independently by Liu et al. and Zendman et al. in 1999, GPR56 (also named TM7XN1) transcripts were found to be expressed differentially in human melanoma cell lines [
86,
87]. Notably, GPR56 was strongly expressed in less metastatic melanoma cells, while markedly reduced in the highly metastatic ones. Therefore, the expression levels of GPR56 transcripts seemed to correlate inversely to the metastatic potentials of melanoma cells. Subsequent studies indeed showed a similar contrary relationship between GPR56 protein expression and tumor metastasis of the melanoma lesions [
31,
32,
33]. Nevertheless, more studies are needed to fully verify the potential role of GPR56 as a negative metastatic marker of human melanoma cells.
Saito et al. found that GPR56 expression was positively regulated by the ecotropic viral integration site-1 (EVI1) transcription factor in acute myeloid leukemia (AML) cells. As such, GPR56 was highly expressed in EVI1
high AML cells that displayed strong cell adhesion and antiapoptotic activities [
79]. GPR56 gene silencing in AML cells resulted in reduced cell growth and increased apoptosis. In addition, Gpr56 was shown to be involved in the normal development and repopulating ability of HSCs in mice. As EVI1 was implicated in regulating the stemness of leukemia cells, GPR56 was suggested as a potential therapeutic target for EVI1
high AML [
79].
Consistent with this, Pabst et al. later identified GPR56 as a reliable leukemia stem cell (LSC) biomarker for most primary human AMLs by using next-generation sequencing and in vivo analyses of LSC frequencies. Moreover, the GPR56 expression level in LSCs was positively correlated with high-risk AML groups and poor clinical outcomes [
85]. Daga et al. subsequently showed that GPR56 is the prominent surface marker of the LSC-enriched CD34
+CD38
− AML cells, while Daria et al. confirmed the association of high GPR56 expression with the inferior prognosis of AMLs [
88]. Experiments involving the adoptive transfer of GPR56-expressing cells significantly promote leukemia development and reduce survival rate in mice. In addition, the inhibition of AML cell engraftment by GPR56-specific Abs further supports a causal link between GPR56 and cancer progression [
79,
84,
85]. In conclusion, GPR56 not only is a valid LSC marker and a disadvantageous prognostic factor of AML but also a potential anti-leukemic therapeutic target.
Liu et al. showed that GPR56 is an independent unfavorable prognostic factor of epithelial ovarian cancer (EOC) by investigating its immunohistochemistry expression in 110 ovarian serous carcinoma samples. They found that the GPR56 expression level was significantly associated with the advanced FIGO (International Federation of Gynecology and Obstetrics) stage and positive lymph node invasion of EOC [
82]. Likewise, Lim et al. examined GPR56 expression levels in the tissue samples of colorectal cancer (CRC) patients by immunohistochemistry and found that the GPR56
high expression group had a lower 5-year overall survival rate than the GPR56
low expression group, suggesting that the GPR56 expression level might be a prognostic indicator of CRC [
89]. Zhang et al. further demonstrated that GPR56 is involved in modulating the plasticity of cancer stem cells (CSC) of CRC to a more drug-resistant phenotype. It was believed that GPR56 promoted drug resistance of CRC by upregulating multidrug resistance protein 1 (MDR1) expression via a RhoA-dependent signaling pathway [
90] (
Table 1).
Table 1. ADGRG1/GPR56 as a cancer marker and/or prognostic factor.
| Cancer Type |
Function |
References |
| Melanoma |
Potential negative metastatic marker/factor |
[31,32,33,86] |
| Acute myeloid leukemia |
Leukemia stem cell marker
Unfavorable prognostic factor |
[79,84,85,88] |
| Epithelial ovarian cancer |
Unfavorable prognostic indicator |
[82] |
| Colorectal cancer |
Unfavorable prognostic indicator
Promote drug-resistant cancer stem cell |
[89,90] |
While the role of GPR56 as unique marker and/or prognostic factor of certain cancer types was strongly implicated, several caveats need to be considered. For example, a systematic evaluation of GPR56 protein expression in human cancer cells and tissues is yet to be conducted. As the splice variants and protein isoforms of GPR56 are not examined in most cancer studies, the possible impact of these receptor variants in the progression of different tumors remains obscure. Thus, the exact GPR56 RNA transcript and protein variants in different stages of tumor development need to be investigated fully. The results of these studies shall provide better insights into the understanding of the role of GPR56 as unique marker/prognostic factor of certain malignancy.
This entry is adapted from the peer-reviewed paper 10.3390/cells10123352