Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Anthropology
The conjunction analysis suggested that viewing high-calorie food cues activated the OFC in both normal-weight people and people with obesity.
- high-calorie food cues
- normal-weight
- obesity
1. Introduction
The prevalence of obesity is problematic and rising in both developed and developing nations [1]. This fact has far-reaching and costly implications, because obesity contributes to the development of numerous diseases (e.g., diabetes, some cancers) [2,3,4], and it is a risk factor for psychiatric disorders (e.g., depression, anxiety) [5]. Not surprisingly, excessive weight has become an increasing threat to healthcare systems [6], and accounts for an estimated 2.8 million deaths per annum worldwide [7]. These statistics have prompted a plethora of research aimed at understanding factors that contribute to the development or maintenance of obesity [8,9,10,11,12].
One contributing factor is the overconsumption of high-calorie or unhealthy foods (e.g., chocolate cake), and underconsumption of low-calorie or healthy foods (e.g., salad), which leads to a positive energy balance and, subsequently, weight gain [13,14,15]. We are currently facing the rise of the ‘obesogenic’ environment [16] where the exposure to food advertisements, and availability of cheap, unhealthy, and energy dense foods has dramatically increased [17,18]. The constant exposure to high-calorie foods and food cues may promote overconsumption by stimulating brain reward and motivation pathways [19,20]. In this vein, using techniques such as functional magnetic resonance imaging (fMRI), a growing number of research has been conducted to investigate neural responses to various forms of food stimuli [21], such as liquid tastants, food odors [22], or visual food cues [23,24]. Moreover, recent reviews have used fMRI-based meta-analysis such as Activation Likelihood Estimation (ALE) [25,26] to evaluate the consistency of findings across these studies [23,24,27,28,29,30,31,32]. For instance, Chen and Zeffiro meta-analyzed 39 experiments with 995 participants and found that taste (e.g, insula), sensory integration (e.g., postcentral gyrus), and reward processing (e.g., amygdala) regions were involved in processing sweet food cues (one kind of high-calorie foods) [30]. With regard to visual food cues, several fMRI-based meta-analyses have also been conducted [18,24,27,28]. For example, an ALE meta-analysis including 12 experiments and 201 participants reported that visual food cues were reliably associated with increased blood oxygen level dependent (BOLD) response in the visual system proper (e.g., the occipital lobe) rather than reward-related brain network (e.g., the orbitofrontal cortex) [28].
None of the aforementioned meta-analyses, however, have investigated which brain regions are concurrently activated in response to viewing high-calorie food cues specifically. Furthermore, most of these meta-analyses only included participants with normal-weight and did not consider individuals with obesity (i.e., body mass index ≥30. A meta-analysis pooling data across relevant fMRI studies would therefore be warranted, as it may help to understand neural responses of viewing high-calorie food cues among people with various weight-status categories (e.g., normal-weight, obesity) and develop better interventions for preventing or reducing overeating and obesity.
2. Brain Response to High-Calorie Visual Food Cues in People with Normal-Weight
For brain activations of viewing high-calorie food cues in participants with normal-weight, the meta-analysis of 39 independent samples (493 foci) identified seven significant clusters (total volume of activation of 10,680 mm3 and maximum ALE value of 0.0713) that covered regions of the bilateral fusiform gyrus, OFC, insula, as well as the right lingual gyrus (Table 1, Figure 1).
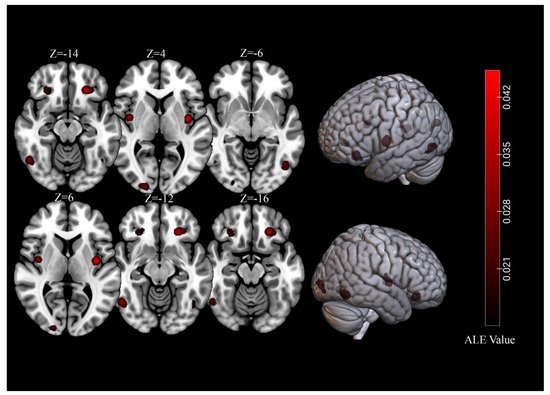
Figure 1. Significant clusters for of viewing high-calorie food cues in samples of individuals with normal-weight.
Table 1. Separate meta-analytic results of significant clusters in individuals with normal-weight or obesity.
| Cluster | Cluster Size (mm3) | Brain Region | Peak Voxel MNI Coordinates | ALE Value (×10−2) | Z | Contributing Samples | |||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | No. | % | |||||
| Normal weight | |||||||||
| 1 | 2080 | L Orbitofrontal Cortex | −24 | 32 | −14 | 4.01 | 6.56 | 9 | 23% |
| 2 | 1600 | R Lingual Gyrus | 20 | −96 | 4 | 2.92 | 5.36 | 8 | 21% |
| 3 | 1568 | L Fusiform Gyrus | −46 | −68 | −6 | 2.73 | 5.02 | 8 | 21% |
| 4 | 1568 | L Insula | −38 | −6 | 6 | 4.53 | 7.13 | 9 | 23% |
| 5 | 1560 | R Fusiform Gyrus | 50 | −60 | −12 | 3.23 | 5.65 | 7 | 18% |
| 6 | 1160 | R Insula | 40 | −4 | 4 | 3.62 | 6.11 | 8 | 21% |
| 7 | 1144 | R Orbitofrontal Cortex | 28 | 32 | −16 | 2.24 | 4.37 | 8 | 21% |
| Obesity | |||||||||
| 1 | 1680 | L Orbitofrontal Cortex | −26 | 34 | −16 | 2.56 | 5.33 | 6 | 35% |
| 2 | 1344 | L Lingual Gyrus | −16 | −100 | −4 | 1.96 | 4.47 | 6 | 35% |
| 3 | 1000 | R Orbitofrontal Cortex | 32 | 28 | −14 | 1.96 | 4.48 | 4 | 24% |
| 4 | 928 | Anterior Cingulate Cortex | 0 | 36 | 14 | 2.15 | 4.75 | 5 | 29% |
Note: L: left, R: right. These presented clusters were significant at a p < 0.001 corrected for multiple comparisons using cluster-level fami-ly-wise error correction at a p < 0.01 (1000 permutations).
3. Core Brain Regions Activated by High-Calorie Visual Food Cues
Our overall results are similar to a previous meta-analysis focusing on the functional neuroanatomy of high-calorie food liquid processing (e.g., sweet liquid) [30].
The amygdala and OFC are connected with each other and frequently activated in food studies. The amygdala is thought to form the core of a neural system for fear processing [99]. However, accumulating evidence indicates that the amygdala also plays a prominent role in mediating positive/reward stimuli processing [100]. These findings have led to the viewpoint that the amygdala’s predominant role may be the detection of and response to motivationally important stimuli [101]. In addition, it was proposed that the amygdala was responsible for forming an “affective tag” to the salient stimuli [102]. Therefore, the amygdala activations that we found in current meta-analysis are likely to reflect the salience and emotional impacts of high-calorie food cues. The OFC receives information from brain regions involved in sensory processing (e.g., insula, fusiform gyrus), affective processing (e.g., amygdala), and memory (e.g., hippocampus), and plays a prominent role in integrating, encoding, and retrieving reward value about stimulus [103]. There is a strong and consistent activation of the OFC in reward-related tasks such as decision-making tasks [104] or cue-reactivity tasks [105]. Further, several studies have shown that the magnitude of activity in this region correlated with pleasantness or tastiness ratings of food/food cues [106,107,108]. Therefore, the OFC activations in current study could reflect the process of monitoring and encoding higher reward value of high-calorie food cues. It should be noted that we did not find that “classical” reward areas such as the nucleus accumbens, putamen, or caudate were involved in processing of high-calorie visual food cues. This is different from a previous meta-analysis which showed that the putamen and caudate exhibited responses to high-calorie liquids [30]. Although comparing across meta-analyses is difficult given differences in included studies, researchers could speculate that reward areas are more likely to be activated when people are eating/tasting high-calorie foods rather than viewing high-calorie food cues.
The insula/frontal operculum has been identified as the primary taste cortex [109,110]. The activation of this taste cortex in response to high-calorie visual food cues may represent memory retrieval of previous gustatory experiences with these palatable foods [23]. In addition, the insula has also been highlighted as a region that plays an important role in craving for drugs (e.g., cocaine) [111] and foods [112]. Therefore, it is also possible that insula activation is the result of high urges to eat in participants exposed to calorie-rich and appetizing food pictures [27].
Researchers also found some evidence that the culmen was activated by high-calorie food cues. Although traditionally considered a major motor structure of the brain, there is evidence that the culmen/cerebellum plays a broader role in homeostatic regulation [113], and shows connections with limbic and reward systems [114]. Given that both the meta-analysis of neural responses to sweet stimuli by Chen and Zeffiro [30] and the current meta-analysis found that the cerebellum increases activity in response to food simulation, future studies and theories of eating behavior may benefit from inclusion of cerebellar influences in their hypotheses forming process.
The remiaining significant clusters found in response to high-calorie visual food cues were located in the occipitotemporal gyrus (the bilateral lingual gyrus and fusiform gyrus, the right middle occipital gyrus). These visual areas consistently respond to multiple drug-related (e.g., alcohol, cocaine, marijuana, tobacco) [115] and gaming cues [116]. Drawing parallels it is conceivable that higher reward salience of higher reward salience of high-calorie food images modulate neural activity in these visual areas, just as drug-related cues do, and leads to different visual processing when compared to control images.
4. Common and Specific Brain Activations between Normal-Weight and Obesity
From an evolutionary perspective, energy-dense foods confer a greater survival advantage for primate species, including humans. From this viewpoint, researchers argued that our species has a natural preference for high-calorie foods [117], which then could be perceived as more rewarding. In light of this, the results of our conjunction analysis–increased activity in the OFC, a reward-related brain area – are not surprising.
The null findings in the contrast analysis between groups of individuals with normal-weight and obesity are different from the conclusions from previous reviews on the topic of cue reactivity in obesity. Past work showed that obesity was related to an enhanced reward/salience response towards (high-calorie) food stimuli [97,98]. It should be noted that these reviews included studies using other stimuli than high-calorie food pictures (e.g., gustatory stimuli, such as chocolate milkshakes), which might lead to different conclusions than our work. Indeed, when only included studies using food pictures, two newly published meta-analyses found similar results to current work [118,119]. For example, Morys and colleagues [118] meta-analyzed 13 studies that investigated group differences (obese vs. normal-weight) in responses to food vs. non-food pictures viewing, and found little evidence for obesity-related differences in brain responses to food cues. In addition, our results are in line with the behavioral literature, as a meta-analysis including 45 published reports did not find evidence for the influence of BMI on food cue reactivity [11]. Taken together, current evidence tends to support that there are no (high-calorie) visual food cue reactivity differences between normal-weight people and people with obesity. Intuitively, this conclusion might contradict some findings of longitudinal studies on the topic of cue reactivity, which found that behavioral and neural responses to food cues predict weight gain [11,120]. However, researchers argued that food cue reactivity is not the only factor that influences food intake and weight gain [9,12,97,118]. For instance, theories proposed that reactions to hyper-palatable food cues might lead to increased food intake and weight gain only in individuals with lower dietary self-regulation, though future research should examine whether this is the case.
This entry is adapted from the peer-reviewed paper 10.3390/brainsci11121587
This entry is offline, you can click here to edit this entry!
