Pea
DDWF1 and
Pra2 are two light regulated genes involved in BR biosynthesis. Pra2 interacts with DDWF1 to regulate the C-2 hydroxylation activity of DDWF1 which converts 6-deoxotyphasterol (6-deoxoTY) and TY to 6-deoxoCS and CS, promoting BR production [
120]. Besides, both
DDWF1 and
Pra2 are dark-induced and light repressed, although how light suppresses the transcriptional level of
DDWF1 and
Pra2 is still unclear. This evidence signifies that light negatively controls BR biosynthesis through arresting
DDWF1 and
Pra2 gene expression in pea. COGWHEEL 1 (COG1), a Dof-type transcription factor, represses light signal in a phyA and phyB dependent manner [
121]. Gain-of-function of
cog1-3D partially suppresses the short hypocotyl of
bri1-5, while loss-of-function of
cog1-6 exhibits short hypocotyl in the light, indicating COG1 positively regulates BR response but negativity controls photomorphogenesis. Biochemical evidence reflects that COG1 stimulates
PIF4/5 gene expression via directly binding to the promoter of
PIF4/
5, which in turn promote the expression of
DWF4 and
BR6ox2 through interacting with the G-box motif presenting at their promoters [
122]. The transcriptional cascade, from COG1 to DWF4 and BR6ox2 which is mediated by PIF4/5, elevates the BR level to allow hypocotyl elongation. Therefore, photomorphogenic repressors COG1 and PIF4/5 function as critical nodes to orchestrate the interconnection between light and BR [
122].
AGB1, an Arabidopsis G-protein β subunit, is known to repress photomorphogenesis for the partial de-etiolation phenotype of
agb1 mutant [
123,
124]. It has been reported that AGB1 represses the expression of
BBX21 and interacts with BBX21 to restrict its transcriptional activity [
125]. In addition, AGB1 interacts with HY5 to inhibit its DNA-binding ability, as well as interacts with PIF3 to protect PIF3 from phosphorylation and degradation [
126,
127]. Both protecting of PIF3 and the inhibitory of HY5 and BBX21 contribute to AGB1-induced skotomorphogenesis. However, the blue light-triggered interplay between CRY1 and AGB1 results in the disassociation of AGB1 from HY5, releasing HY5-mediated genes expression and photomorphogenesis [
126]. In the red light, photoactivated phyB competitively binds with AGB1 which removes the protection of PIF3, leading to the phosphorylation and degradation of PIF3 and facilitate photomorphogenesis [
127]. It is worth to mention that the role of AGB1 in the light signal may be similar to COP1 which also contains a domain homologous to the β subunit of trimeric G proteins [
31]. In addition, AGB1 interacts with BES1 to improve the ratio of dephosphorylated to phosphorylated BES1, as well as synergistically modulates the expression of BES1 targets such as
CPD,
DWF, and
SAUR family genes required for cell elongation, supporting that photomorphogenic repressor AGB1 is a positive regulator of BR response (
Figure 3) [
128]. Thereby, AGB1 is identified as a junction between light and BR pathways. However, whether AGB1 acts as a bridge to connect BES1 with BBX21 and HY5 to coordinate light and BR cascade remains elusive. BBX32, the last member of BBX family of proteins in Arabidopsis, is a photomorphogenic repressor based on its role in promoting hypocotyl elongation and inhibiting anthocyanin accumulation [
129]. A recent study indicates that BBX32 interacts with PIF3 to promote BR-mediated cotyledon closure during transition from dark to light. In addition, BBX32 associates with BZR1 which elevates the expression of
BBX32 to form a positive feedback regulation. Thus, these data suggest that BBX32 acts as a node to integrate light and BR signaling to modulate cotyledon closure during de-etiolation [
130].
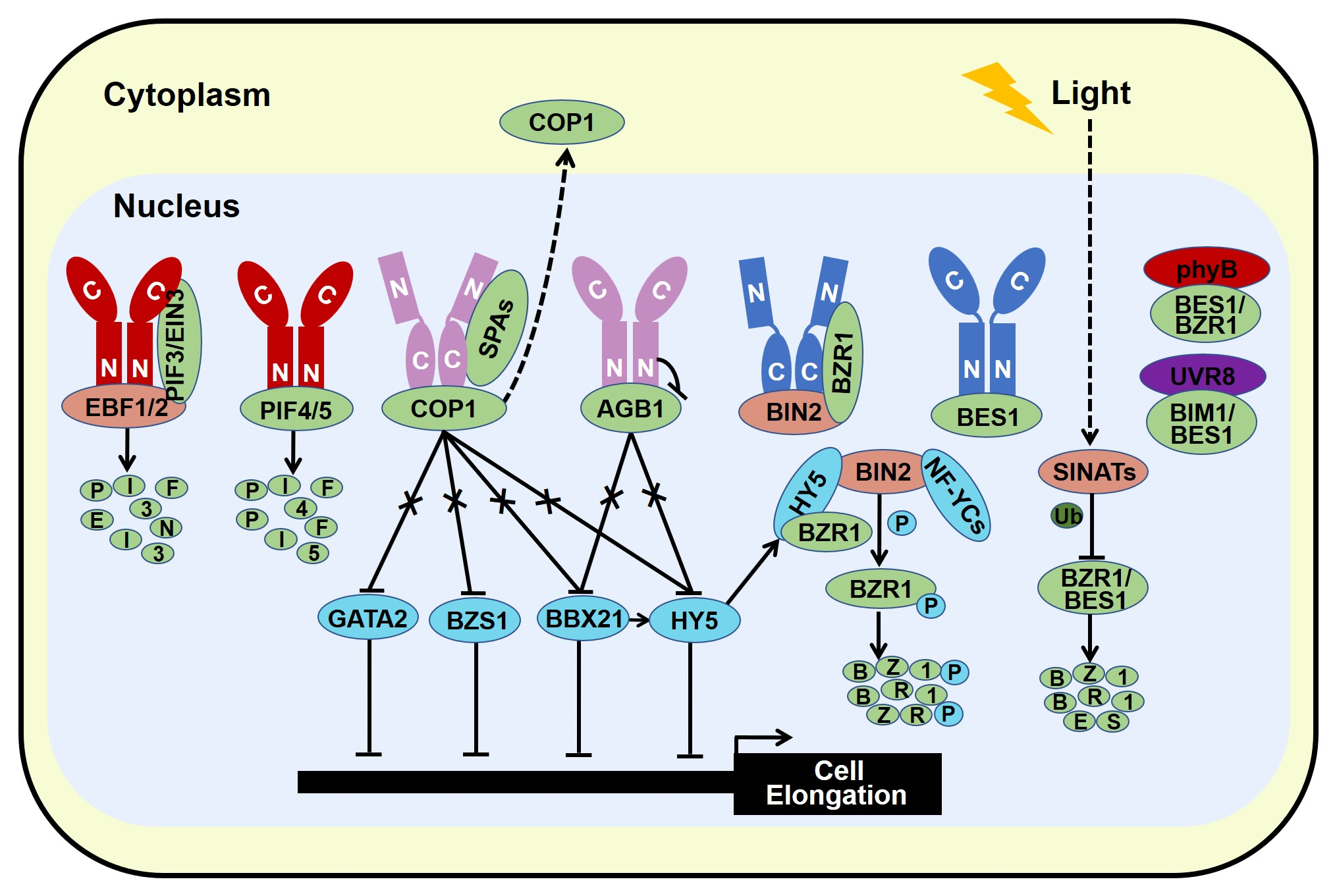
The stability of BES1 and BZR1 is tightly controlled by several E3 ligases. MORE AXILLARY GROWTH LOCUS 2 (MAX2), a subunit of SCF E3 ligase, is critical for strigolactone signaling. BES1 has been reported to interact with MAX2 which promotes BES1 degradation, leading to the inhibitory of shoot branching [
131]. PLANT U-BOX 40 (PUB40), a U-box ubiquitin E3 ligase, is responsible for proteasome-mediated degradation of BZR1 in a root-specific manner [
132]. SINA OF ARABIDOPSIS THALIANA (SINATs), RING finger E3 ubiquitin ligases, are degraded in the dark, while light promotes the accumulation of SINATs which directly interact with BES1 and BZR1. Biochemical evidences signify that light-stabilized SINATs prefer to bind dephosphorylated BES1 and capture BES1 for ubiquitination and degradation to arrest hypocotyl elongation and BR cascade (
Figure 4) [
133]. In addition, the phosphorylated BZR1 serves as the substrate of COP1 for degradation in darkness, increasing the ratio of dephosphorylated to phosphorylated BZR1. The relatively accumulated active form of BZR1 results in more active dimers with either dephosphorylated BZR1 or PIF4 to enhance BR response and hypocotyl elongation [
134]. Similar with phosphorylated BZR1, COP1 is responsible for the degradation of phosphorylated BES1 in the dark [
133]. Therefore, stabilized SINATs mediate the ubiquitination and degradation of dephosphorylated BES1 in the light to promote photomorphogenesis, whereas dark-activated COP1 degrades phosphorylated BZR1 and BES1 to increase the relatively ratio of active form of BZR1 and BES1. Both dark-reduced SINATs protein level and dark-stimulated COP1 activity contribute to the high ratio of active form of BZR1 and BES1 in the nucleus, allowing the BR response and obstructing photomorphogenesis.
Figure 4. Light and BR share common regulatory components to optimize plant growth and development.
Previous data elucidate that BZR1 and BES1 interact with skotomorphogenic-promoted transcription factor PIF4 to synergistically regulate their target genes that are required for cell elongation (
Figure 3) [
8]. Recently, it is documented that BZR1 and PIF4 promote the gene expression of GROWTH REGULATED FACTOR 7 (
GRF7) and
GRF8 to repress chlorophyll biosynthesis. Besides, GRF7, BZR1, and PIF4 interact with each other to precisely modulate the greening process via regulating genes involved in chlorophyll biosynthesis. Thus, GRF7-BZR1-PIF4 module orchestrates light and BR signaling pathway to enhance seedling survival during de-etiolation [
135]. In addition, BLUE-LIGHT INHIBITOR OF CRYPTOCHROMES 1 (BIC1), which is identified as a repressor of flowering and photomorphogenesis via inhibiting CRY2 phosphorylation, acts as a transcriptional coactivator of BZR1 and PIF4 for the activation of BR-responsive genes to promote hypocotyl elongation. Collectively, BIC1-BZR1-PIF4 complex integrates light and BR response to coordinate plant growth [
136]. Moreover, PIF4 along with PIF3 have been reported to serve as substrates of BIN2, which facilitate the phosphorylation and degradation of PIF3 and PIF4 [
11,
137]; however, COP1-SPA complex interacts with PIF3 and interferes with the BIN2-PIF3 interaction, leading to the accumulation of PIF3 and thus to facilitate skotomorphogenesis [
137]. Furthermore, high temperature-promoted nuclear-localized BZR1 binds to the promoter of
PIF4 to promote its gene expression and thermomorphogenesis [
138]. Thus, the BIN2-BZR1-PIFs regulatory module integrates hormonal and environmental signals to coordinate plant growth and development.
2.2. Photomorphogenesis-Promoting Factors Repress Brassinosteroid Signal
Recent studies have revealed that blue light receptor CRY1 blocks the BES1 DNA-binding activity and limits its target gene expression via specifically interacting with the physiologically functional dephosphorylated BES1, thereby promoting photomorphogenesis and impeding BR response [
13]. Besides, photoactivated phyB interacts with dephosphorylated BES1 to repress its transcriptional activity, allowing plants to balance light and BR signaling [
15]. Similar to BES1, blue light-excited CRY1 and red light-excited phyB interact with the DNA-binding domain of BZR1 to reduce its DNA-binding ability and confine the expression of its target genes [
14,
16]. Moreover, blue light-activated CRY1 interacts with BIN2 to strengthen the physical interaction between BIN2 and BZR1, promoting the phosphorylation of BZR1 and inhibiting its nuclear accumulation, ultimately leading to the impedance of hypocotyl elongation [
14]. Therefore, visible light exploits the phyB-BES1/BZR1, CRY1-BES1, and CRY1-BIN2-BZR1 regulatory module to obstruct BR signaling to optimize plant photomorphogenesis. In addition, BR response is also inhibited by UV-B radiation. Similar to phyB and CRY1, UVR8 physically interacts with BES1 INTERACTING MYC-LIKE1 (BIM1) and dephosphorylated BES1. Upon UV-B radiation, monomerized UVR8 and UVR8-BES1 complex are accumulated in the nucleus, leading to the repression of BES1 DNA-binding activity and its target gene expression [
12]. Moreover, BES1 directly binds to the promoters of
PFG MYBs to repress gene expression and constrain flavonol biosynthesis, while broad-band UV-B limits the transcription of
BES1 in a UVR8 independent manner, leading to the accumulation of flavonol and UV-B tolerance [
18]. Thereby, UV-B light employs UVR8-BES1/BIM1 interaction and BES1 to orchestrate UV-B signal and BR signal in fine-tuning plant growth.
Moreover, HY5, the famous photomorphogenesis-promoting transcription factor, has been proved to specifically interact with dephosphorylated form of BZR1 and reduce its transcriptional activity, consequently leading to repress the expression of BZR1-controlled genes that are related to cotyledon opening. Ectopic expression of HY5 decreases the abundance of BZR1. Thus, HY5 not only represses the transcriptional activity of BZR1 but also attenuates its protein stability to promote cotyledon opening [
139]. However, the physical connection between HY5 and BES1 in the regulation of seedling morphogenesis remains to be dissected. Recently, HY5 has been documented to interact with BIN2 to enhance the kinase activity of BIN2 possibly via improving BIN2 Tyr200 autophosphorylation, ultimately promoting seedling photomorphogenesis (
Figure 4) [
17].
GATA2 is a transcription factor that regulates genes in response to light via the essential GATA light-response promoter element (LRE) [
140]. Knock down of
GATA2 lines show long hypocotyl in the light, while overexpression of
GATA2 confers short hypocotyl and open cotyledon in the dark resembling the light-grown seedlings, indicating that GATA2 is a photomorphogenesis-promoting factor [
140]. In the dark, COP1 interacts with GATA2 to facilitate its ubiquitination and degradation, meanwhile, BR confines the transcript level of
GATA2 through the directly binding of BZR1 on the promoter of
GATA2 [
140]. Thus, both the low RNA and protein level of GATA2 contribute to etiolation in darkness. Under light treatment, attenuated COP1 activity in the nucleus induces the protein accumulation of GATA2 which in turn negatively regulates its own transcription via the directly binding to the promoter of itself. Such opposite regulatory of transcript and protein levels form a negative feedback loop to maintain a proper protein level of GATA2 for the right photomorphogenesis. Both BZR1 and GATA2 impede
GATA2 gene expression, whether BZR1 interacts with GATA2 to cooperatively regulate the transcription of
GATA2 needs further investigation. Similar with GATA2, knockdown of
BZR1-1D SUPPRESSOR 1 (BZS1) displays slightly long hypocotyl in the red and blue light, while overexpression of
BZS1 exhibits de-etiolation phenotype in the dark. Such genetic data imply that BZS1 promotes photomorphogenesis [
9]. BZS1, also known as BBX20, shows extensive sequence homology to BBX21 [
141]. Gain of function of BZS1-D is a dominant suppressor of BR hypersensitive mutant
BZR1-1D, reflecting BZS1 is a negative regulator of BR signal. Further studies indicate that BZS1 is degraded in the dark via a COP1-dependent manner, whereas BZR1 represses the transcriptional level of
BZS1 which antagonistic controls gene expression involved in light and BR signaling [
9]. Thus, light-inactivated COP1 results in BZS1 accumulation to promote photomorphogenesis, whereas BR-activated BZR1 reduces
BZS1 gene expression to alleviate the inhibition of BR cascade. Taken together, GATA2 and BZS1 act as hubs to integrate light and BR signaling (
Figure 3 and
Figure 4). As the closet homolog of BZS1, BBX21 is critical for photomorphogenesis via enhancing the gene expression and transcriptional activity of HY5 [
28,
87], however the role of BBX21 in BR signal is vague. Further analysis of the function of BBX21 will add new insights into the crosstalk between light and BR signals.
NUCLEAR FACTOR-Y C PROTEINS (NF-YCs) are subunits of NF-Y heterotrimeric complex that regulate target gene expression by specifically binding to the CCAAT-box-containing promoters [
142]. NF-YCs not only inhibit BR biosynthesis by directly targeting the promoter of the BR biosynthesis gene
BR6ox2, but also interact with BIN2 to promote its Tyr200 autophosphorylation and protein stabilization, resulting in inhibiting the BR signaling pathway during light-controlled hypocotyl growth [
19]. Therefore, photomorphogenic-promoting regulators NF-YCs repress BR biosynthesis and signaling to regulate seedling development.
2.3. MicroRNAs Integrate Light and Brassinosteroid Signals
MicroRNAs (miRNA) are a group of small, non-coding endogenous RNA molecules (sRNA) that play crucial roles in plant growth and development. Some microRNAs have recently been reported to regulate seedling morphogenesis partially through the regulation of light and brassinosteroid signaling. MicroRNA396a regulates several members of the GRF family at posttranscriptional level. Overexpression of miR396a develops a constitutive photomorphogenic phenotype in the dark, including shorter hypocotyls, opened cotyledons, and highly accumulated Pchlide. Consistently,
grf1/4/7/8 quadruple mutant shows dramatically lower greening rate compared to the wild type, and also exhibits a de-etiolation phenotype in the dark. In addition, GRF7/8, BZR1, and PIF4 interact with each other to form a tripartite module, which positively regulates cell elongation related genes, but negatively controls Pchlide biosynthesis related genes. Collectively, MiR396 represses gene expression of
GRFs at posttranscriptional level, resulting in promoting photomorphogenesis and repressing brassinosteroid signaling during seedling de-etiolation [
135]. Brassinosteroid up-regulates the expression of
MiR395a which might repress
GUN5 expression, leading to promote the root development [
143]. In addition, brassionsteroid decreases the localization of AGO1 at the endoplasmic reticulum (ER), which inhibits the miRNA-mediated translational repression, resulting in increasing the protein levels of miRNA target genes [
144]. MicroRNA osa-miR1848 targets
OsCYP51G3 for suppression at posttranscriptional level.
OsCYP51G3 encodes an obtusifoliol 14α-demethylase which positively regulates phytosterol and brassinosteroid biosynthesis. Increased osa-miR1848 and decreased
OsCYP51G3 expression cause a BR deficiency phenotype, including dwarf plants, semi-sterile pollen grains, and erect leaves. Thus, osa-miR1848 negatively regulates brassinosteroid biosynthesis to control plant architecture, which has potential to be utilized in rice breeding by adjusting leaf angle, plant height, and seeds quality [
145].
Overall, in the absence of BRs, the constitutively activated BIN2 not only phosphorylates and inactivates BZR1/2, but also phosphorylates PIF3/4 which result in the degradation of PIF3/4, consequently to promote photomorphogenesis [
11,
137]. In the presence of BR, inactivated BIN2 not only relieves BZR1/2, but also releases PIF3/4. The stabilized and activated PIF3/4 and BZR1/2 act in concert to promote downstream gene expression to stimulate skotomorphogenesis/etiolation. In the dark, activated COP1 targets phosphorylated BZR1 and BES1 for ubiquitination and degradation, leading to the high ratio of active form of BZR1 and BES1 [
133,
134]. Besides, COP1-SPA complex interacts with PIF3 and blocks the BIN2-PIF3 interaction, resulting in the accumulation of PIF3 [
45,
68]. Moreover, brassinosteroids receptors perceive signal at the cell surface and deliver it to BZR family transcription factors and PIF transcription factors in the nucleus respectively. Both the high ratio of dephosphorylated BZR1/2 and high protein level of PIFs contribute to facilitate skotomorphogenesis (
Figure 3). However, in the light, photoreceptors employ light-promoted transcription factors such as HY5, BBX21, BZS1, and GATA2 to stimulate photomorphogenesis by directly regulating hypocotyl-related gene expression or enhancing the kinase activity of BIN2 or any other unknown mechanisms. Besides, photoreceptors also directly interact with COP1-SPAs complex, AGB1, PIFs, and BZR1 family transcription factors to repress their activity and/or stability to facilitate photomorphogenesis (
Figure 4).