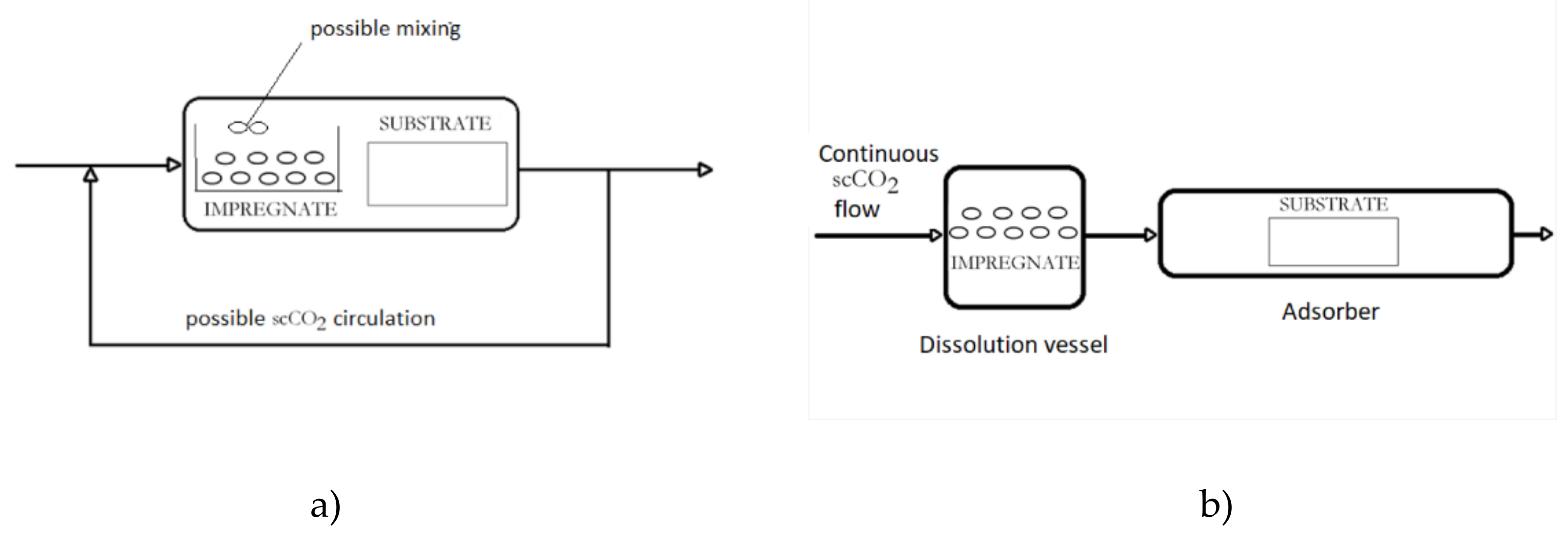
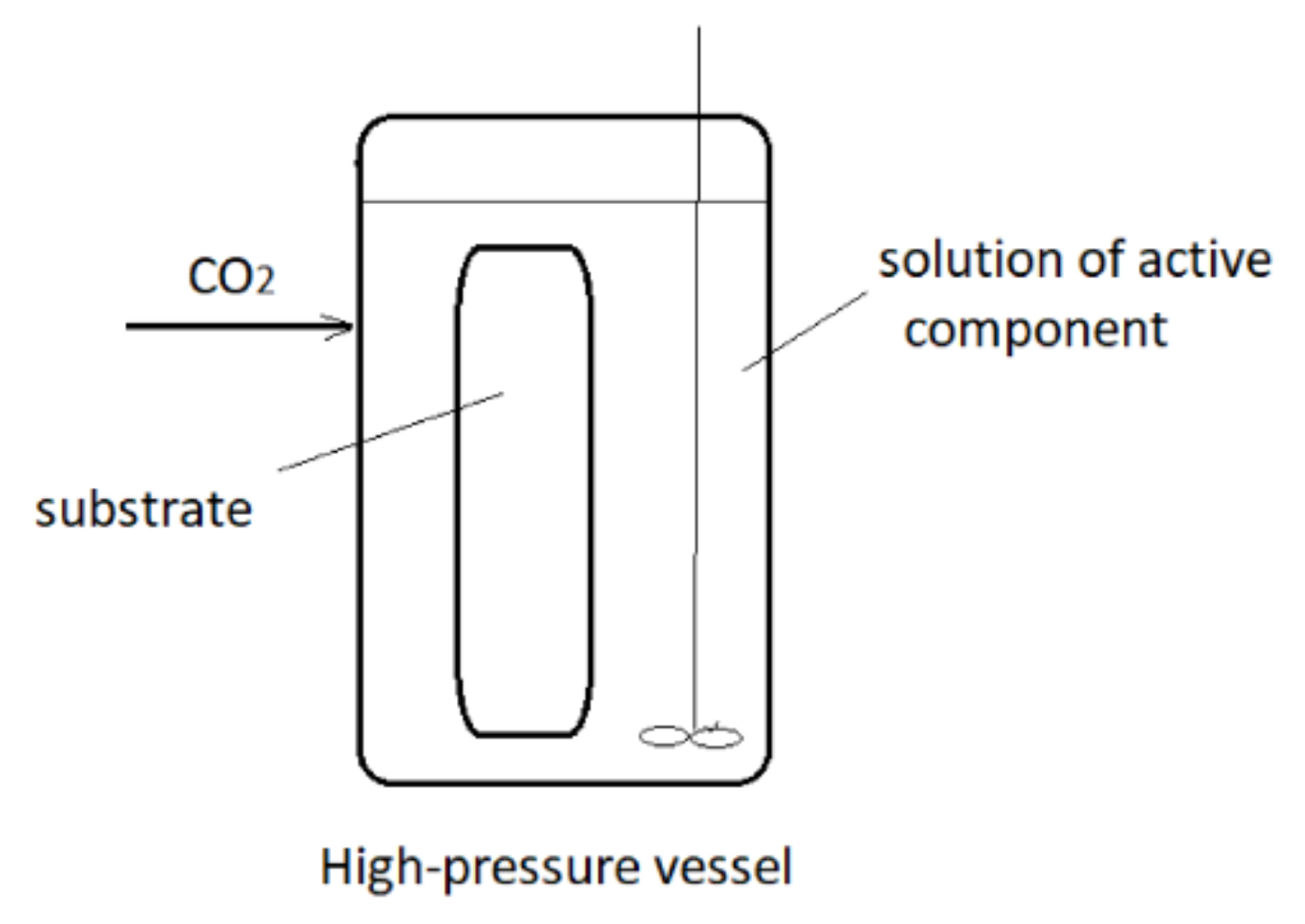
3. Supercritical Assisted Impregnation (SAI) and High-Pressure Assisted Impregnation (HPAI)
These techniques may be applied to the impregnation of active substances, which are less soluble in scCO2 or not soluble at all. In those processes, an active component is dissolved in an appropriate liquid solvent, and the liquid phase is brought to contact with a solid to be impregnated in the presence of supercritical or high-pressure carbon dioxide (hpCO2—CO2 under high pressure but not in the supercritical region). A simplified presentation of the process is presented in Figure 2. In this way, good transport properties of carbon dioxide in a liquid or supercritical state promote the contact between the liquid and solid. Quite often, swelling of the solid surface occurs, which also promotes the impregnation process. In the process, a considerably smaller quantity of the liquid phase to dissolve the active component is usually employed in comparison to the conventional impregnation from liquids. Also, a cosolvent may be added to scCO2 to enhance the interaction between the active substance and the supercritical phase. The main difference between the SSI and SAI techniques is the following: in SAI, a contact between 3 phases exists (solid substrate, supercritical phase, and liquid phase) no matter what is the solubility of the active component in scCO2. The active principle may be dispersible or soluble in scCO2. Unlike, in the case of SSI, the substrate is in contact with the supercritical phase only.
Figure 2. Simplified presentation of Supercritical Assisted Impregnation (SAI)/High-Pressure Assisted Impregnation (HPAI) process.
The first and essential application of HPAI was in leather tanning
[7]. Leather is produced when an impregnate (tanning agent—usually chromium-III-salt) reacts chemically with collagens in pretreated animal hides in an aqueous solution. Leather manufacturing conventional process is exceptionally intensive concerning the consumption of resources, and an estimated overall amount of about 14 million m
3 of wastewater per year is generated worldwide
[7]. In the new process, the skins are contacted with a tanning solution and subsequently contacted with hpCO
2 (>3 MPa) in rotating tanning drums. CO
2 is partly diffusing into the skin and in the tanning solution. The leather of high quality is obtained already after 2 h of contact with CO
2. There is no wastewater generation in the new process. In comparison to conventional method, consumption of the tanning agent is decreased for more than 50% and there is no need for the addition of sodium salt
[7]. Further in text, results on the implementation of HPAI and SAI in the production of novel antibacterial materials will be presented.
Mölders et al.
[8] applied carbon dioxide in a liquid (12 MPa, 20 °C) and supercritical state (12 MPa, 40 and 80 °C) to impregnate polycarbonate with silver nitrate as an antibacterial agent. The experiments were performed in a batch mode in a high-pressure view cell but also scaled up in a high-pressure vessel of 2 L. The samples were submerged in an ethanol solution of silver nitrate, heated, pressurized, and impregnated for 10 min. In parallel, submerging tests were performed under atmospheric pressure. Impregnation assisted by scCO
2 was superior in comparison to the impregnation in liquid CO
2 and far more superior than submerging at ambient pressure, providing silver content of around 23.4 mg/kg polymer. HPAI with liquid CO
2 provided silver content of 2.4 mg/kg polymer while submerging under atmospheric pressure and 80 °C resulted in a content of 0.2 mg/kg polymer. The samples impregnated by both supercritical and liquid carbon dioxide showed strong antimicrobial activity against
E. coli. Abrasion as well as UV-radiation and led to a loss of antimicrobial activity of the samples impregnated at 20 °C. However, the samples impregnated at 80 °C resisted the tests. The leaching of the samples was analyzed to determine the toxicity on humans, and the toxicity could not be confirmed
[8]. These excellent results opened a way towards production of antibacterial surfaces which could be applied to many elements in hospitals such as doorknobs, switches, handrails, buttons, surfaces for placement of medical devices, etc.
The subsequent studies deal with the development of a novel class of antibacterial mats based on carbon nanomaterials and silver nanoparticles (NPs)
[9][10]. Carbon nanotubes and nanofibers wrapped by silver NPs were fabricated with the assistance of scCO
2 [9]. The SAI process was performed with the ethanol solution of the carbon materials, the silver precursor (AgNO
3), and glucose as a reducer at 12 MPa and 65 °C for 3 h. The TEM and SEM images revealed that carbon nanotubes/AgNPs hybrids possess a preferable assembled structure. Experimental results demonstrated considerable antibacterial activity of tested materials against
E. coli [9]. In the following study, Haldorai et al.
[10] reported results on graphene oxide treatment with silver NPs in the presence of scCO
2 to produce a material with photocatalytic and antibacterial activity. Graphene oxide was treated in an ethanol solution with AgNO
3 and glucose as a reducer at 12 MPa and 65 °C for 3 h. The graphene oxide modified with silver NPs displayed an excellent visible-light photocatalytic performance in degrading Rhodamine 123 dye and acetaldehyde as well as significant antibacterial activity against
E. coli,
S. aureus, and
Listonella anguillarum [10].
Based on the available literature survey, results on the SAI and HPAI applications are scarce but impressive. These techniques are a powerful tool yet to be applied to the design of novel materials. In the next section, the review will present combined processes of SAI/SSI and polymerization in scCO2, which opened possibilities for the design of unique antibacterial mats.
4. Supercritical Solvent Impregnation or Supercritical Assisted Impregnation Coupled with Polymerization in scCO2
The subsequent studies deal with the application of composite polymers known as interpenetrating polymer network (IPN)
[11] in biomedical purposes. Solvent-free IPNs can be produced using scCO
2 [11]. In this process, one or more monomers are dissolved or dispersed in supercritical or near-critical carbon dioxide and brought to contact with a polymer to be impregnated (SSI or SAI). The polymerization and crosslinking of monomers can be performed by a radical starter that can be impregnated into the polymer matrix simultaneously with the monomer(s). The polymerization reaction may be triggered by the temperature increase in the supercritical conditions upon the impregnation, consequently leading to the formation of IPN. Because there is no chemical bonding between the polymer and the network (between two polymers), each material retains its individual properties in the blend. This allows for a variety of applications for the novel type of materials synthesized in an environmentally friendly way
[11]. The different behavior of the polymers in IPN in combination with the solvent-free appearance of the final product makes these materials especially attractive for the design of medical devices.
Steffenson et al.
[12][13] demonstrated that silicone elastomers used in catheter production could be modified to form an IPN material with a poly(2-hydroxyethyl methacrylate) (PHEMA)-based hydrogel. Extruded silicone
[12] and poly(dimethylsiloxane) (PDMS) silicone elastomer
[13] were impregnated with (2-hydroxyethyl) methacrylate (HEMA) and ethylene glycol dimethacrylate (EGDMA) in the presence of cosolvent(s) and a radical starter in scCO
2. The impregnation was performed at 40 °C and pressures 20–25 MPa for a time from 20 min to 16 h, depending on the contact between phases. The polymerization proceeded at 75 °C and 30–36 MPa for 3 h. Fabricated IPN materials retained mechanical properties similar to those of the original silicone elastomer while acquired the ability of the hydrogel to swell in aqueous media. PHEMA content was in the range of 13–38% (
w/
w). It was shown that the hydrogel formed an interconnected hydrogel network in aqueous media when the content of PHEMA was at least 25%. The optimized IPN material was loaded with the antibiotic ciprofloxacin, and the resulting drug release inhibited bacterial growth of
S. aureus when placed on agar
[12]. In the further study
[13], it was demonstrated that samples containing 25% (
w/
w) hydrogel loaded in a 5 mg/mL ciprofloxacin medium inhibited
S. aureus growth upon incubation in broth with high efficacy for 29 days whereby no biofilm was observed on the material. These substantially significant results opened a possibility for the design of novel medical devices for long-term clinical use.
In the next study, Stenger et al.
[14] produced IPN catheters by the polymerization and crosslinking of PHEMA in silicone elastomer in scCO
2 as previously described
[12][13]. The system was loaded with dicloxacillin alone or in combination with thioridazine and tested against methicillin-sensitive
S. aureus and MRSA. The drug-loaded IPN material was proven to be effective in in vitro experiments. Moreover, the IPN catheters were tested in a novel porcine model of central venous catheter-related infection, in which they were found to decrease the frequency of infection significantly
[14].
The results presented on the preparation and application of IPN materials with controlled release of antibiotics are of the utmost importance bearing in mind that bacterial colonization with subsequent biofilm formation constitutes a severe and frequent problem associated with the use of many polymer materials commonly applied for medical devices
[13]. Urinary tract infections are the most frequently occurring nosocomial infections
[15]. During the long-term use of catheters, the risk of urinary tract infections increases rapidly over time and reaches 50% after 7–10 days
[13][16]. To illustrate the significance, in the USA, approximately 250,000 of vascular catheter-related bloodstream infections occur annually associated with a mean hospital length stay of 22 days, increasing the hospital cost from US$ 3000 to 56,000 per patient, and with mortality rates of 12–25% for critically ill patients
[13][17][18].
The use of carbon dioxide as a polymerization reaction medium has been investigated continuously since it is a green solvent with many advantages over conventional solvents
[19][20]. Correia et al.
[19] reported a method to obtain biocompatible 2-oxazoline-based oligomers quaternized with different amines using scCO
2 as a reaction medium. Oligo(2-methyl-2-oxazoline) and oligo(2-bisoxazoline) quaternized with N,N-dimethyldodecylamine were shown to be very efficient biocidal agents showing fast killing rates against
S. aureus and
E. coli. In a further study, Correia et al.
[21] presented a novel approach to the design of antibacterial materials by combining plasma technology, SSI, and polymerization in scCO
2. In this study
[21], oligo(2-methyl-2-oxazoline) quaternized with N,N-dimethyldodecylamine was grafted to a chitosan (CHT) scaffold. Chitosan scaffolds were prepared with the freeze-drying method, and subsequently, their surface was activated by argon plasma treatment. Upon the activation, the scaffolds were subjected to the SSI with the monomer 2-isopropenyl-2-oxazoline for 24 h at 18 MPa and 40 °C. After this grafting step, another monomer, together with an initiator, was introduced into the system, and the polymerization took place at 18 MPa and 65 °C for 20 h. In the final step, a tertiary amine was added to the reactor and the reaction was performed at 18 MPa and 40 °C for 20 h. The material obtained efficiently killed
S. aureus and
E. coli cells upon direct contact and prevented bacterial adhesion to the materials surface and biofilm formation. The material was shown to be suitable for water purification over ten cycles of reuse, efficient within minutes of contact and without leaching to the water
[21].
Cationic antimicrobial peptides are promising antibacterial agents
[19] and, as presented, can be synthesized and grafted to solid carriers in scCO
2 [21]. Their mechanism of action is based on electrostatic forces and subsequent interaction between the cationic peptide and the anionic lipopolysaccharide in outer membrane of Gram-negative bacteria or the negatively charged teichoic acids attached to the thick layer of peptidoglycan present in the surface of Gram-positive bacteria
[19][22]. It is believed that bacteria cannot develop resistance to these antibacterial polymers because the mechanism of action depends on the fundamental characteristics of the microbial cytoplasmic membrane. Therefore, the development of resistance would require bacteria to change their membrane structure completely
[19][23].
5. Supercritical Foaming
Dissolution of scCO
2 in polymers may increase chain mobility and induce polymer swelling in amorphous and semi-crystalline polymers, at the same time decreasing their melting point under the supercritical conditions
[24]. Optionally, a cellular structure of the polymer matrix (foam) can be formed by inducing phase separation with a pressure and/or temperature change. Supercritical foaming was previously mentioned in connection to SSI of PCL
[25]. In this part, it will be commented more since it is not connected with the SSI technique only, as it will be seen from the next example.
García-González et al.
[26] reported results on the preparation of PCL-chitosan scaffolds containing vancomycin as an antimicrobial agent by scCO
2 foaming, aimed for bone regeneration purposes. The foaming was performed from solid dispersions of PCL, chitosan, and the antibiotic. Powdered mixtures with different PCL, vancomycin, and chitosan contents were introduced into cylindrical Teflon molds, compacted, and exposed to scCO
2 at 40 °C and 14 MPa for 1 h with subsequent decompression under the CO
2 flow rate of 1.8 g/min. The obtained scaffolds showed a suitable combination of morphological (porosity, pore size distribution, and interconnectivity), and vancomycin release behavior, as well as the biological properties (cell viability and proliferation, osteo differentiation, and tissue-scaffold integration). The scaffolds sustained vancomycin release in PBS for more than two weeks and showed considerable antibacterial activity against
S. aureus and
E. coli [26]. This study exemplifies a method for the incorporation of a substance poorly soluble in scCO
2 (vancomycin) into polymeric foams. Subsequent studies relate to the incorporation of a scCO
2 soluble substance into polymer matrix by foaming and SSI as a one-step process.
Ivanovic et al.
[24] reported results on the impregnation and foaming of PCL and polycaprolactone-hydroxyapatite (PCL-HA) composites with thymol in scCO
2 for obtaining functional porous scaffolds. The effect of scCO
2 sorption kinetics on the swelling, foam morphology, and thermal behavior of the PCL and PCL-HA materials was studied, whereby sorption isotherms were determined using a magnetic suspension balance at 10–30 MPa and 35–40 °C and thermal properties using high-pressure differential calorimetry (HP-DSC) at pressures 4.6–17.0 MPa. In the next step, SSI of PCL and PCL-HA with thymol was performed simultaneously with the foaming to produce scaffolds with antimicrobial properties and controlled microstructure. The pressures in the range 13–17 MPa and 10% of HA were proven to be favorable for the creation of scaffolds with satisfying foam microstructure (mean pore size ∼200–300 µm), filler distribution, and thymol loadings (12–18%)
[24].
Milovanovic et al.
[27] prepared foams loaded with thymol in a one-step SSI-foaming process from amorphous, medical grade poly(D,L-lactic acid) (PLA), and poly(D,L-lactic-co-glycolic acid) (PLGA). The impregnation performed with different CO
2 densities (273–815 kg/m3) and short processing times (2 and 4 h) enabled thymol loading of 0.92–6.62%. The process was optimized for each polymer to obtain stable microcellular foams upon the system decompression. The highest thymol loading (6.62%) was obtained for the copolymer PLGA, whereby the sample exhibited controlled thymol release within 72 h in media having pH values from 1.1 to 7.4
[27].
6. Supercritical Drying of Metal-Carrying Gels
Synthesis of metallic nanoparticles is of great importance for the application in catalysis, electronics, and optics and for the design of materials with antibacterial properties
[28]. The preparation of metal colloids by the reduction is a simple reaction. Still, the control of particle size, shape, and dispersion stability requires careful control of the synthetic conditions because the process is sensitive to balances between nucleation and crystal growth
[28][29]. One approach to facilitate both the synthesis control and immobilization is the use of porous materials as reaction medium, which might be a hydrogel
[28]. The hydrogel can further be transformed into an alcogel by the solvent exchange and subsequently to an aerogel by supercritical drying resulting in a highly porous added value material for a wide range of applications
[28][30][31]. Aerogels are characterized by the small bulk densities (0.017–0.021 g/cm
3), low thermal conductivities, big surface area (200–800 m
2/g), and proven capability for the controlled release of incorporated substances
[30][32][33][34].
Cai et al.
[28] synthesized silver, gold, and platinum nanoparticles in the cellulose hydrogel by hydrothermal reduction by the cellulose itself (for silver at 80 °C for 24 h) or by adding a reductant (for gold and platinum). To produce aerogels, the water of metal-cellulose hydrogels was exchanged to ethanol, and two-step batch drying in carbon dioxide was applied. First, the ethanol was replaced with liquid CO
2 at 5.3 MPa and 4 °C for 6 h and then supercritical drying took place at 10 MPa and 40 °C for 0.5 h, with subsequent slow decompression. The aerogels obtained were characterized by the high transmittance, porosity, and surface area as well as good mechanical strength
[28].
Raman et al.
[30] reported results on the synthesis of calcium-alginate aerogels augmented with zinc and silver for potential application in wound healing. By the combination of high-pressure gelation (room temperature, 50 MPa, for 24 h) and supercritical drying with a continuous flow of scCO
2 (at 50 °C and 12 MPa for 2 h and under 20 g/min CO
2 flowrate), hybrid Ca–Zn particles as well as hybrid Ca–Zn–Ag aerogel monoliths and particles were produced. The metal ions were released into supernatants upon the aerogels swelling in aqueous solutions in the amounts needed for a wound dressing
[30].
In the subsequent study
[31], pectin-TiO
2 nanocomposite aerogels were prepared via the sol-gel process, consecutive solvent exchange step, and supercritical drying. The drying was performed at a temperature and pressure in ranges 50–60 °C and 11–13 MPa, respectively, for 5 h and with the scCO
2 flow rate of 0.2 kg/h. In the presence of TiO
2 nanoparticles, mechanical, thermal, and antimicrobial properties (against
E. coli) of pectin-based aerogels were improved in comparison to the control ones. Thus, the aerogels may provide antibacterial protection and, to some extent, thermal protection due to the low thermal conductivity and may have a potential application in packaging for sensitive items
[31].
7. Other Methodologies Applied to the Development of Antibacterial Materials
In this part, more ideas for the utilization of the extraordinary properties of supercritical fluids in the design of antibacterial materials will be presented. In the recent study, Li et al.
[35] presented results on the synthesis of the hybrid Cu
2O/TiO
2 nanocomposites with the enhanced photocatalytic antibacterial activity against
Acinetobacter baumannii.
A. baumannii is Gram-negative bacteria, widespread, and multidrug resistant often found in intensive care unit, where it causes intra-hospital infections including sepsis, urinary tract infections, ventilator-acquired pneumonia, and wound infections
[35][36]. In this study
[35], a stable combined p-n Cu
2O/TiO
2 heterojunction was prepared by a supercritical solvothermal process in ethanol. The supercritical solvothermal process is regarded as a powerful tool for the synthesis of heterojunction materials with considerable advantages over conventional methods. Compared to the physical mixture, aqueous reduction, photochemical, and hydrothermal routes, all accompanied with weak combination, nonuniform size distribution, and easy aggregation, the application of supercritical fluids can provide a stable combination between Cu
2O and TiO
2 with the uniform dispersion and small crystal size resulting in the large special surface areas with a mesoporous structure and the expended visible-light absorption
[35]. It is believed that the high rate of crystal nucleation without the easy crystal growth is the consequence of the high temperature and pressure applied in the supercritical state
[35][37][38]. Cu
2O/TiO
2 composites were synthesized in supercritical ethanol at 243 °C and 6.4 MPa. In this process, Cu(NO
3)
2·5H
2O and
tetrabutyl titanate were dissolved into absolute ethanol and kept at the operating conditions for 70 min to complete the synthesis. The bactericidal activity of the 5.0% Cu
2O/TiO
2 sample in the case of
A. baumannii was 100% under the visible-light irradiation within 30 min. Moreover, the 5.0% Cu
2O/TiO
2 nanocomposite displayed the significant visible-light antibacterial activities (up to 100% mortality in 30 min) against other pathogenic bacteria including
P. aeruginosa,
E. coli, and
S. aureus. Based on the experimental findings, it was presumed that the Cu
2O/TiO
2 composite first led to the leakage of K
+ ion with the disrupted permeability of the cell membrane and then induced the formation of inorganic compounds from the cell decomposition. The composites were shown to be durable due to the stable p-n Cu
2O/TiO
2 heterojunction obtained under the supercritical conditions. The durability and photocatalytic antibacterial activity of the composites present significant potential for the application in disinfection
[35].
Bhartia et al.
[39] used scCO
2 for grafting semiconductor surfaces with monolayers of alkylthiols. Hydrogen-terminated semiconductor surfaces were exposed to alkylthiols dissolved in scCO
2 at 100 °C and 10 MPa for the chemical reaction and establishment of the strong and nonpolar Si–S surface bond. The deposited monolayer on oxide-free silicon was stable, dense, and able to passivate the surface for more than 50 days (10 times than the conventional methods) without any oxide formation in the ambient atmosphere. The material resisted cell proliferation on the surface for more than 15 days and, besides the application in electronics, is envisaged for biomedical and antimicrobial applications. The inert nature of CO
2, as the ideal contamination-free isolated processing environment for grafting better-quality monolayers, allowed for the production of superhydrophobic and bio-resistant surfaces
[39]. In this environmentally free process, drawbacks of conventional technologies are overcome and the product of better quality is obtained.
Katayama et al.
[40] used scCO
2 to induce large pleat-like wrinkles on the surface of cotton fibers as support for nanoparticles. The cotton was immersed into the water first, and the treatment with scCO
2 followed. The process parameters were optimized to produce the appropriate wrinkles. The favorable conditions were found to be a temperature of 40 °C, the pressure of 20 MPa, the contact time of 60 min, and a fast decompression rate of 0.80 MPa/min
−1. It is assumed that the wrinkles occur due to the different degasification rates from the inner and surface parts of the fiber during the fast decompression. The material obtained was proven to be a suitable support for TiO
2 nanoparticles of average 35 nm in diameter without the presence of binders
[40].
Cuadra et al.
[41] used scCO
2 as an antisolvent to prepare a new adduct of isonicotinamide with copper(II) propanoate, a ligand complex with strong fungicidal properties. The precipitation was performed by introducing an ethanol solution of the components through a 100-µm nozzle into scCO
2 at 40 °C and 10 MPa at a flow rate of 1 mL/min. ScCO
2 dissolves in the ethanol, consequently decreasing the ligand complex solubility and leading to the precipitation. Applying the supercritical antisolvent (SAS) technique, crystals 100-fold smaller than those obtained by slow evaporation were produced, indicating a considerable bioavailability enhancement
[41].
Imbuluzqueta et al.
[42] employed liquid CO
2 (10 MPa, 25 °C) as an antisolvent to produce a novel bioactive hydrophobic gentamicin-filled carrier. In this process, gentamicin was ion-paired with the anionic surfactant Bis(2-ethylhexyl) sulfosuccinate sodium salt (AOT) to obtain a hydrophobic complex (GEN–AOT). The solution of GEN–AOT in acetone was sprayed through a hollow cone nozzle into the CO
2, resulting in the precipitation due to the antisolvent effect and allowing for GEN-AOT micronization. In a further step, the encapsulation of the obtained complex in PLGA nanoparticles was performed by the emulsion solvent evaporation method. The procedure provided NPs with GEN–AOT encapsulation efficiency of 100% and sustained release of the drug over 10 weeks. It was also shown that neither ion pairing, supercritical fluid processing, nor encapsulation in polymeric NPs affected the bactericidal activity of gentamicin against
E. coli [42].
Saelo et al.
[43] applied Rapid Expansion of a Supercritical Solution into a Liquid Solvent (RESOLV) process to obtain caffeic acid phenethyl ester (CAPE) nanoparticles. The mixture of CAPE, ethanol, and scCO
2 at 17.3 MPa and 50 °C was expanded through a nozzle at 80 °C into distilled water. The obtained CAPE NPs were incorporated into methylcellulose films in the process of film preparation by the solvent casting method. Films containing 0.5% of CAPE NPs exhibited antimicrobial properties against
P. aeruginosa,
C. albicans, and
Listeria monocytogenes [43].
Varona et al.
[44][45] applied high-pressure techniques PGSS (Particles from Gas Saturated Solutions) and PGSS-drying to encapsulate lavandin (
Lavandula hybrida) essential oil known for its antibacterial and antiviral properties. Carrier materials investigated were soybean lecithin, n-octenyl succinic anhydride (OSA) modified starch, PCL, and polyethylene glycol (PEG). PGSS was applied to the oil encapsulation into PCL
[44] and PEG
[45]. In this process, the lavandin oil and polymer were filled together in a high-pressure cell and intensively mixed in the presence of scCO
2 for 2 h (the polymer was in a molten state) to reach phase equilibrium. Then, the mixture was depressurized, and due to the rapid expansion through a nozzle to ambient pressure, small particles were formed. The driving force for particle formation is the strong cooling as a consequence of the Joule Thomson effect produced during the expansion. It results in the polymer solidification and a covering layer formation around the essential oil droplets. PGSS-drying was applied to the oil encapsulation into OSA modified starch and soybean lecithin
[44][45]. In this process, an oil-in-water emulsion was prepared in which the essential oil constitutes the dispersed phase and OSA-starch/soybean lecithin acts as a surfactant. The emulsion saturated with CO
2 was contacted with the scCO
2 in a static mixer and subsequently expanded through a nozzle. The expansion facilitated the formation of extremely fine droplets which dried very fast, while the polymer solidified encapsulating the essential oil. The results showed an enhancement of the antibacterial activity of lavandin oil against
E.coli,
S. aureus, and
Bacillus cereus by the encapsulation due to the protection and control release provided by the carrier
[44]. PGSS processes may provide polymer particles of micron size, filled with an antimicrobial agent, and suitable for spraying onto different surfaces.
Recent studies
[46][47][48][49] demonstrated the feasibility of scCO
2 application in liposome production. Conventional methods of liposome production suffer from drawbacks, such as the difficulty of controlling particle size distribution, micrometric dimensions, low stability, and high solvent residue
[48]. To overcome these deficiencies, Santo et al.
[46] and Trucillo et al.
[47] developed a continuous supercritical assisted process called SuperLip (Supercritical assisted Liposome formation), characterized with good control of particle size distribution, possibility to produce liposomes on a nanometric or micrometric level, liposome stability of over one year, and solvent residue in liposomes lower than FDA limits
[47]. The SuperLip process was successfully applied to the production of liposomes with antimicrobial activity loaded with vancomycin
[47], amoxicillin
[48], ampicillin, and ofloxacin
[49]. In this process, an ethanol solution of phospholipids is brought to contact with scCO
2 at 10 MPa and 40 °C in a saturator vessel first. The expanded liquid ethanol-scCO
2 mixture is subsequently introduced into a high-pressure formation vessel operating at the same temperature and pressure conditions as the saturator. An aqueous solution with an active substance is injected through a nozzle into the formation vessel as well. The atomized droplets of water solution are quickly captured by the phospholipids contained in the fluid phase, creating lamellae around the inner core containing the drug. This is the key step of the scCO
2-assisted process and an inversion of the traditional liposome production
[48]. These inverted micelles, falling in a water bulk formed at the bottom of the vessel, are covered by a second lipids layer, completing the double-layer structure. The results showed that it was possible to control particle size distribution at the nanometric level, with an encapsulation efficiency of the drug up to 84%
[48].
In the following study, Trucillo et al.
[50] applied two scCO2-assisted techniques to load alginate aerogels with liposomes containing amoxicillin. The SuperLip process was used first to obtain amoxicillin loaded liposomes. In the next step, liposomes were entrapped in alginate hydrogels. After the water replacement with ethanol, obtained alcogels were subjected to supercritical drying to obtain aerogels. The results demonstrated that ampicillin release time from these meta-carriers was about four days or twice its release time from liposomes alone
[50].