Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Positron emission tomography (PET) imaging with 18F-fluorodeoxyglucose (FDG) represents a method of detecting and characterizing arterial wall inflammation, with potential applications in the early assessment of vascular disorders such as atherosclerosis.
- atherosclerosis
- 18F-sodium fluoride
- NaF
- 18F-fluorodeoxyglucose
- FDG
- PET
1. Introduction
Atherosclerosis is the leading cause of cardiovascular diseases (CVDs) [1]. Globally, 31% of all deaths in 2016 were caused by CVD, of which 85% were due to heart attacks or strokes [2]. Atherosclerotic changes of the vasculature can be detected through different imaging techniques and can be divided into two main categories centering either on the degree of stenosis or on plaque composition. Previously, the clinical focus has been on measuring the degree of the stenosis and the subsequent physiological effect. To assess the cardiac physiology, radionuclide ventriculography, often referred to as a MUGA (multiple-gated acquisition) scan, where a gamma camera following an injection of radioactively labeled red blood cells is used to measure the left ventricular ejection fraction (LVEF). Echocardiography is another technique that uses sound waves to produce images of the heart and, also, allows for the assessment of LVEF. Myocardial perfusion scintigraphy has traditionally used single-photon emission computed tomography (SPECT), but more recently, positron emission tomography (PET) is also increasingly used in the diagnosis of ischemic chest pain and for the evaluation of known coronary artery disease (CAD).
2. Role of FDG in Atherosclerosis
Atherosclerosis, or atherosclerotic cardiovascular disease, is a chronic condition characterized by arterial stiffening due to the buildup of cholesterol plaques on vessel walls [65]. Endothelial cell dysfunction is believed to underlie the pathogenesis of atherosclerosis. In brief, hypertension and hyperlipidemia contribute to the upregulation of endothelial cell adhesion molecules [66]. The resultant recruitment of inflammatory cells propagates the inflammatory cascade, including platelet activation, deposition of lipid plaques, smooth muscle proliferation, and, ultimately, vessel micro- and macrocalcifications [13]. Progressive enlargement of these plaques throughout the body leads to a spectrum of debilitating cardiovascular conditions, including peripheral artery disease, ischemic stroke, coronary artery disease, and acute myocardial infarctions [67]. These conditions represent a major cause of morbidity and mortality both in the Unites States and worldwide [68,69,70]. Therefore, effective strategies to identify atherosclerotic disease early in the disease pathogenesis, as well as to quantify the extent of disease burden, are alluring.
Conventional imaging modalities, including ultrasonography, CT, and MRI angiography, are widely used clinically to visualize large symptomatic plaques but are limited in their ability to assess the early stages of atherosclerosis [71,72]. In contrast, molecular imaging offers a tantalizing opportunity to examine the pathological hallmarks of atherosclerotic disease at the microscopic level [73]. As discussed previously, FDG demonstrates remarkable sensitivity and specificity for inflammatory lesions. Further, FDG was postulated to be effective in the identification of the inflammatory precursor lesions that precede calcific atherosclerotic disease. Yun et al. first examined vascular FDG uptake in 137 patients who underwent FDG-PET scanning [23]. They observed that over half of the subjects demonstrated vascular FDG uptake, with a greater prevalence among older individuals. Further studies demonstrated that vascular inflammation as assessed by FDG was associated with proinflammatory molecular and cellular markers of atherosclerosis [24,25,26].
It is becoming apparent, however, that the association between FDG uptake and risk factors associated with disease progression may be less straightforward than originally postulated. Yun et al. demonstrated in a later study of 156 patients that intravascular FDG uptake was significantly related to age and high cholesterol but not other cardiovascular risk factors, including cigarette use, diabetes mellitus, hypertension, and obesity [27]. Other researchers have demonstrated similar results using a variety of protocols and parameters to further characterize the clinical impact of arterial FDG uptake [3,28,29,30,40,41,42,74,75]. While the results of these studies are challenging to compare directly, in general, FDG uptake demonstrates a clear association with age but only a vague relationship with other risk factors [31,32]. For example, Pasha et al. measured the tissue-to-background ratio and weighted-average mean standardized uptake value to examine 76 patients who underwent FDG-PET/CT imaging and found that patients with cardiovascular risk factors had increased FDG uptake in the aorta but not in the peripheral (i.e., femoral and iliac) arteries [30]. Due to this variability, careful interpretation and clinical correlation should be applied to focal vascular FDG uptake.
Moreover, FDG has been found to demonstrate a low specificity for the future development of calcifications [33,34,35,36,76]. The uptake of FDG by endothelial cells and smooth muscle cells increases in hyperinflammatory states such as cancer, thereby potentially obfuscating the localization and quantification of FDG uptake due to atherogenic activity [5]. Meanwhile, stable disease, which may present with substantial plaque burdens but minimal or variable inflammation, similarly obscure the FDG-PET findings, what Meirelles et al. described as the “waxing and waning” effect [37]. That is, while focal FDG uptake is frequently observed in atherosclerotic disease, it has not been clearly associated with the structural manifestations of atherosclerosis identifiable by CT. Interestingly, arterial macrocalcification detected by CT has been shown to regress in angina patients over a 2-year period, suggesting that structural changes associated with atherosclerosis may also not be stable for measurements over time [43]. While further longitudinal studies should be performed to corroborate the results, the variability of FDG uptake during atherosclerosis progression further challenges the temporal use of FDG-PET/CT.
Numerous studies have investigated the relationship between FDG uptake within vulnerable plaques and the risk for future cardiovascular outcomes [31,77,78]. For example, the FDG uptake at plaques found in carotid arteries was found to be higher in patients who experienced early recurrent strokes [79]. An association between high FDG uptake and plaques with high-risk morphological features has been confirmed histologically as well [80]. Despite the apparent positive results, however, there remains significant challenges to using FDG-PET to study atherosclerotic plaques. First are the technical challenges intrinsic to FDG-PET, such as low specificity and resolution; the accurate measurement of FDG uptake in plaques can be hampered by high physiological myocardial FDG uptake, small diameter of the arteries, and cardiac motion [5,81]. A decreasing myocardial FDG uptake requires prior adherence to a high-fat, low-carbohydrate diet, which can be hard to follow for patients [82,83]. Furthermore, the absence of FDG uptake in the plaque may not always indicate a truly negative result, as it could be due to the insensitivity of PET to detect small foci of the FDG uptake [84].
Another argument against the use of FDG-PET for studying atherosclerotic plaques is the limited clinical significance of the vulnerable plaque; it is well-known that plaques can rupture without any preceding or warning symptoms, the morphology of plaques detected by imaging modalities can vary over periods of time, and plaque lesions that rupture are often previously characterized as non-culprits. Furthermore, only a small number of vulnerable plaque ruptures cause actual symptomatic events. Therefore, FDG-PET should not be limited in its scope to examining specific plaques. Rather, it should be used for deriving atherosclerotic burdens measured from FDG uptakes in broader anatomical structures and vessels [74].
The last general limitation of FDG that must be mentioned is its inability to elucidate the precise cellular mechanism of disease progression and its relationship with organs of high intrinsic glucose uptake. Despite this, FDG-PET has been used to rationalize the mechanistic relationship between CVD and neuropsychiatric conditions through bone marrow and spleen involvement [85,86]. For instance, it has been found that a high FDG uptake in the amygdala correlates with CVD events, arterial inflammation, and FDG uptake in the bone marrow and spleen, which was taken to rationalize that stress may lead to CVD events through the increased production of inflammatory cells from the hematopoietic stem cell niche [38]. However, the main metabolic activity of the bone marrow that accounts for a high FDG uptake is the production of red blood cells, which varies widely among subjects of different ages, rather than the generation of inflammatory cells [87,88,89]. Similarly, FDG uptake in the spleen is known to differ based on the clinical context [90]. Therefore, only relying on FDG-PET to draw specific cellular mechanisms and causal relationships between CVD and organs of high natural FDG uptake should be avoided.
In the current state of research, the prognostic value and implementation of FDG-PET for the assessment of the atherosclerotic risk remain to be further tested. There has been no clear association between the FDG uptake and CT calcium burden, nor a prospective study in noncancerous patients that correlates an increased FDG uptake with adverse cardiovascular outcomes [91]. The CAMONA (Cardio-vascular Molecular Calcification Assessed by 18F-NaF PET/CT) study involving 50 patients with angina pectoris and 89 healthy controls, for instance, revealed no significant correlation of FDG uptake in the thoracic aorta and 10-year Framingham Risk Score (FRS) [34]. Additionally, the variability in the protocols and reporting outcomes further confounded the implementation of FDG-PET. A review of 49 articles using FDG-PET to evaluate atherosclerosis inflammation revealed 53 different acquisition protocols and 46 methods of quantify the tracer uptake. Standardization and harmonization of the method, therefore, remains an essential step to be taken before using FDG-PET in clinical practice [92].
3. Role of NaF in Atherosclerosis
Over the past decade, increasing attention has turned toward NaF-PET/CT to detect vascular microcalcifications. Unlike FDG, NaF is not taken up by metabolically active tissues such as the myocardium, which allows NaF-PET to have a greater sensitivity and less background uptake than FDG for the assessment of CVDs [76]. In their methodological piece, Irkle et al. demonstrated that NaF has demonstrated a sensitivity for calcification in the vascular tissue, thereby lending itself well to atherosclerotic disease [93]. Research using vascular NaF-PET/CT has demonstrated that coronary; pulmonary; and peripheral (i.e., aorta, carotid, iliac, and femoral) artery NaF uptake is significantly correlated with a number of cardiovascular risk factors, including age, BMI, diabetes, hypertension, hyperlipidemia, and cardiovascular events; however, it is not associated with smoking and is variably associated with sex [41,44,45,46].
Evidence points toward NaF uptake as a significant clinical metric for atherosclerosis (Figure 1). Kwiecinski at al. and Kitagawa et al. both found that focal coronary NaF uptake on the index scans significantly correlates with the incidence of myocardial infarction [47,48]. As such, findings on NaF-PET/CT may therefore serve as a tool to assess the future risk of atherosclerosis complications. Other studies by Rojulpote et al. and Patil et al. have correlated the NaF uptake of vital and laboratory values such as blood pressure and the triglycerides-to-high-density lipoprotein ratio, respectively [49,50]. In addition, NaF uptake has been associated with widely used clinical scores for cardiovascular disease burden, including the Framingham Risk Score, atherosclerotic cardiovascular disease (ASCVD) risk scores calculated by the Pooled Cohort Equation, and CHADS2/CHADS2-VASc [34,51,52,53,54].
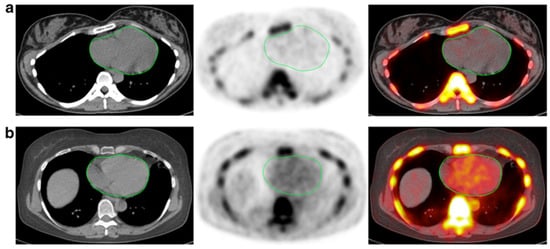
Figure 1. CT, NaF-PET, and fused NaF-PET/CT images of clinically normal (a) 25- and (b) 61-year -old subjects’ hearts. Green line delineates the region of interest around the heart analyzed to calculate the global cardiac calcification scores, which are 12,492.44 (a) and 18,424.70 (b). Despite the relatively increased NaF uptake in the PET scan of the subject’s heart (b), there is no visible calcification in the corresponding CT scan. The disparity between two modalities alludes to CT-visible macrocalcification as end-stage disease process, while NaF uptake may reflect early pathological, molecular changes (from Raynor et al. [94] with permission).
It is clear that molecular imaging will play a major role in atherosclerotic imaging in future clinical practice (Figure 2). Recent evidence has pointed toward NaF as a more useful clinical tool than FDG in the evaluation of atherosclerotic disease, particularly for a high plaque burden. In an examination of 19 multiple myeloma patients who underwent NaF- and FDG-PET/CT scans, Li et al. found that increased NaF uptake was associated with increased plaque density, while the inverse was true for FDG [55]. With regard to the pathological development of atherosclerotic plaques, a number of studies have observed that vascular NaF uptake is greater in high-risk lesions than stable plaques in the carotid and coronary arteries [14,56,57]. In addition, Ishiwata et al. recorded the initial arterial NaF uptake in the abdominal aorta and common iliac arteries and then tracked the atherosclerotic disease progression using CT. They observed that, on index scans, NaF uptake was greater in noncalcified than calcified lesions, perhaps reflecting active plaque deposition; however, the initial NaF findings did not correlate with the disease burden determined by CT alone during follow-up at 1 to 2 years [55,58].
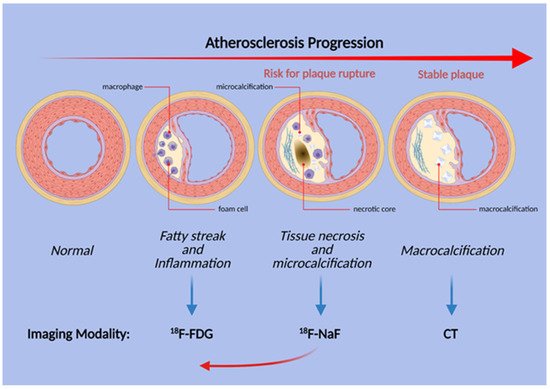
Figure 2. Schematic illustration of the stages of atherosclerosis in the coronary arteries. Uptake of both FDG and NaF is evident before the structural changes are visible, but inflammation and FDG uptake does not necessarily precede microcalcification. Thus, NaF uptake may be present earlier than previously thought (red arrow).
Regardless, there exists a paucity of longitudinal, prospective research utilizing repeat NaF-PET scanning to examine the development and progression of NaF-avid lesions and vessel wall calcifications [95]. There currently has not been any human studies establishing a clear link between arterial NaF uptake by macrocalcification and the subsequent transformation into CT-detectable macrocalcification; the clearest association with early NaF uptake and corresponding coronary macrocalcification was demonstrated using an Ossabaw miniature swine model for metabolic syndrome [19]. Therefore, the development of well-powered, prognostic studies conducted with longitudinal design remains necessary to fill this void and confirm the potential of NaF-PET for the clinical assessment of atherosclerosis [91].
Another parameter that should be examined with caution in PET research design is the use of the target-to-blood pool ratio (TBR), which is derived by dividing the raw standard uptake value (SUV) to the venous blood pool SUV [25]. This derivation attempts to calibrate for the assumed background tracer activity in the blood but currently remains a controversial and even unreliable method. For instance, no clear biological rationale is offered for its use. Furthermore, TBR calculations can introduce large variability to the data, since the venous blood pool SUV is often minimal and affected by wide-ranging factors such as the venous blood flow rate, blood cell uptake, and individual differences in FDG clearance [96]. Blomberg et al. highlighted the unreliability of the TBR method when the authors found that the TBR values calculated at 1, 2, and 3 h after tracer administration were inconsistent [39].
4. Alavi-Carlsen Calcification Score (ACCS)
In lieu of the TBR method, global assessment of the major vessels, called the Alavi-Carlsen Calcification Score (ACCS), may offer significant advantages for using PET imaging to study diffuse CVD activity [97]. The Alavi-Carlsen Calcification Score method of global assessment contrasts with the focal approach, which is limited to specific sites such as atherosclerotic plaques in the coronary arteries [81]. A limitation of measuring the focal uptake in such small vessels arises from the insufficient resolution of most PET scanners, which has the potential to underestimate the associated radiotracer uptake [59,98]. Evaluating the major vessels could overcome this limitation, considering that calcification in the thoracic aorta is shown to strongly correlate with the coronary artery score [99,100].
As such, the ACCS global assessment examines atherosclerosis in its appropriate context as a diffuse, systemic disease that exerts differential effects in various parts of the affected arteries [97,101]. The score is derived from measuring the total tracer uptake in structures such as the entire body, major vessels, or specific organs such as the heart in the form of average SUV over a broad segment of the body. It allows for the measurement of the atherosclerotic burden in the early stages of the disease progression, unlike the method of measuring plaque uptakes that occur in later phases. The regions of interest (ROIs) can easily be defined based on gross structures seen in CT or the cardiac silhouette in 3D. Since the score is measured based on clearly defined and delineated anatomical boundaries, it is less subject to human bias and variations in measurements, even allowing for artificial intelligence (AI)-based approaches with a reproducibility of 100% [91].
The ACCS global assessment approach has been employed to demonstrate that patients with multiple myeloma have a higher uptake of NaF in the thoracic aorta and whole heart, as measured by the target-to-background ratio (TBR) compared to a matched control group [60]. Similarly, the CVD risk factors, such as total cholesterol in patients with type 2 diabetes mellitus, have been shown to be associated with increased NaF uptake when measured as the global TBR in the femoral arteries [61]. A retrospective analysis of 86 healthy controls and 50 patients with persistent chest pain revealed using the ACCS approach of NaF uptake in the whole heart as measured by the mean standardized uptake value (SUVmean) was higher in patients compared to the control subjects and could be employed to retrospectively predict the patient status [62]. Overall, these studies demonstrate the suitability and potential of quantifying disease risk through global assessment, of which the latter has now become an attractive option that is quick and easy to perform, especially using artificial intelligence-based processing [63,64,97,101].
5. Other PET Tracers in Atherosclerosis
The most used radiotracer in PET imaging is FDG, which has well-studied roles in atherosclerosis imaging. However, FDG has several limitations. FDG accumulates in all cells that metabolize glucose, and a high physiologic myocardial uptake obscures the uptake due to the presence of macrophages in atherosclerotic plaques. 68Ga-DOTATATE is a tracer that was originally intended for the improved detection of somatostatin receptor 2 (SSRT2)-positive neuroendocrine tumors. SSTR2 is also expressed on plaque macrophages; hence, it has the potential to visualize vulnerable plaques. Tarkin et al. [102] tested the efficacy of 68Ga-DOTATATE compared to FDG in 42 patients with atherosclerosis, and 68Ga-DOTATATE was shown to differentiate culprit lesions from non-culprit lesions better than images obtained by FDG. In addition, its degree of uptake correlates with the Framingham cardiovascular risk score.
Translocator protein (TSPO) ligands expressed on the macrophage in the process of plaque formation can be targeted by the C-PK11195 tracer. In an animal model, Laitinen et al. [103] demonstrated that tracer uptake was higher in inflamed than in noninflamed plaques but that other healthy structures of the artery wall also had prominent uptake, limiting its potential utility.
The expression of C-X-C motif chemokine receptor 4 (CXCR4) and its endogenous ligands and C-X-C motif chemokine 12 (CXCL12) can be found in cardiac myocytes and fibroblasts [104]. According to Hu et al., there is an upregulation of CXCR4/CXCL12 in response to hypoxia in myocardial infarction, which, in turn, initiates the process of recruitment of cardioprotective cells to protect the myocardium from reperfusion damage [105]. Recently, several studies have demonstrated promising results of the possibility of using CXCR4-directed 68Ga-Pentixafor PET/CT imaging to evaluate atherosclerotic plaque lesions [106,107,108]. These prompt further studies to compare CXCR4-directed 68Ga-Pentixafor PET/CT imaging with imaging using FDG. Kircher et al. conducted a retrospective study to compare the performance between CXCR4-directed 68Ga-Pentixafor PET/CT and FDG-PET/CT in detecting the atherosclerotic lesion, showing that the former was able to visualize more plaque lesions than the latter. Apart from macrophages, CXCR4 could be expressed in thrombocytes, T cells, and smooth muscle cells, representing 68Ga-Pentixafor might be able to detect early-stage lesions without the setting of marked inflammation [109]. As discussed in an earlier section, FDG uptake is only associated significantly with age. A study conducted by Weiberg et al. successfully established that CXCR4-directed 68Ga-Pentixafor uptake has a marked association with different cardiovascular risk factors, including age, arterial hypertension, and history of smoking [110]. The ability of 68Ga-Pentixafor to detect lesions earlier and its association with cardiovascular risk factors make it a promising alternative for FDG imaging.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11122234
This entry is offline, you can click here to edit this entry!
