Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Toxicology
Dibenzo-α-pyrone (DAP) is the basic scaffold of a group of naturally occurring chemicals. From one angle, the gastrointestinal metabolites urolithins are regarded as beneficial, while from the other, the emerging mycotoxin alternariol and related fungal metabolites are evaluated critically with regards to potential hazardous effects.
- urolithins
- mycotoxins
- estrogenicity
- microbiome
1. Introduction
Dibenzo-α-pyrone (DAP, Figure 1A) is the basic scaffold of a group of naturally occurring chemicals, which are mainly formed by microbial species, such as bacteria or filamentous fungi. When substituted with multiple hydroxy groups, those metabolites belong to the chemical class of polyphenols, from which many representatives are regarded as beneficial for human health, mostly due to anti-oxidative and chemopreventive effects [1]. This also applies to some polyphenolic DAP derivatives. For example, urolithin A (UA, Figure 1B), a metabolite formed by ellagitannin-degrading gut bacteria, is extensively researched and marketed as a health-promoting agent in the scope of functional foods [2] or even as a therapeutic agent to improve muscle health [3].
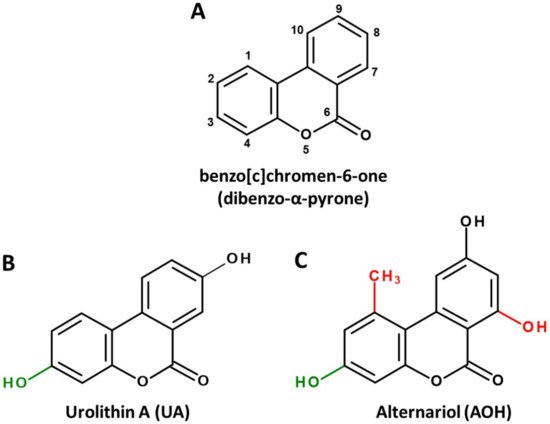
Figure 1. Chemical structures of the basic DAP scaffold (A), as well as two signature representatives of natural DAP derivatives: the bacterial polyphenol metabolite urolithin A (B) and the Alternaria mycotoxin alternariol (C). The hydroxy group at C1 (marked green) is a common feature of most natural DAPs. Methylation of C5, as well as hydroxylation of C11 (marked red), are common in biosynthesized DAPs but are not featured in ellagitannin biodegradation products.
However, there seems to be another side to the story. DAPs biosynthesized by food-contaminating molds, such as the mycotoxin alternariol (AOH, Figure 1C), are regarded as potential carcinogens due to their ability to damage the DNA and to potentially induce endocrine-disruptive effects [4,5].
2. Microbial Sources and Associated Structural Peculiarities
There are isolated reports of DAP derivatives being formed by plants [8], but the two main ways that they are produced in or from food commodities are (a) the complete biosynthesis as secondary metabolites of molds or (b) the biodegradation of ellagitannins by intestinal bacteria. The biosynthesis pathway is described mainly for filamentous fungi, particularly the genus Alternaria. A polyketide synthase encoded by the pksJ gene was found to be critical for the production of the two most prevalent DAPs, AOH and alternariol 9-methyl ether (AME) by Alternaria alternata [9]. Furthermore, the production of these and similar DAPs was also reported in other Alternaria [10], Acremonium [11], Cephalosporum [12] and Hyalodendriella spp. [13], all described as endophytic molds. Mycogenic DAPs are frequently reported in mold-contaminated grains, fruits, vegetables, etc., and are regarded as food contaminants [14]. As data suggest that they could be responsible for potentially toxic effects, but there are no regulations for maximum contamination levels yet around the globe, AOH and AME are considered to belong to the class of emerging mycotoxins [15].
On the other hand, DAPs that derive from the biodegradation of ellagitannins are uniformly referred to as urolithins. After ingestion, ellagitannins are hydrolyzed by bacteria carrying tannase enzymes to yield ellagic acid [16], which is further catalyzed by a currently unidentified lactonase/decarboxylase enzyme to the 3,4,8,9,10-pentahydroxy-DAP, urolithin M-5 (UM5). From the latter, all other urolithins are formed by subsequent dehydroxylation reactions that are catalyzed by currently unidentified enzymes [2]. However, a few bacterial species that are able to carry out at least a part of these reactions were already discovered. Gordonibacter pamelae and Gordonibacter urolithinfaciens, two species belonging to the strictly anaerobic family of Eggerthellaceae, were reported to decompose ellagic acid and perform dehydroxylations to sequentially yield UM5, urolithin M-6 and urolithin C (UC), the latter being the final metabolite [17]. Recently, another Eggerthella species, Ellagibacter isourolithinfaciens, was isolated from a human gut microbiome and observed to be capable of further dehydroxylating UC to isourolithin A [18,19]. Another study found Bifidobacterium pseudocatenulatum INIA P815 to produce UA and UB under certain growth conditions [20]. In complex microbiomes obtained from human feces, high interindividual differences were observed in the activity of the human microbiome, which allows for its stratification into three main groups [2]. Metabotype 0 (accounting for approximately 10–15% of the population) does not produce urolithins from ellagic acid. In urolithin producers, the final metabolites are either UA (metabotype A) or isourolithin A and urolithin B (UB) (metabotype B) [21].
Notably, the common precursor molecule UM5 predetermines that DAPs deriving from ellagic acid breakdown are only substituted with hydroxyl groups and are not functionalized at C1, C2 and C7 (Figure 1B). This is in stark contrast to biosynthesized DAPs, where substitutions at those positions, particularly the methylation of C1 and the hydroxylation of C7, are the norm (Figure 1C). In addition, based on current knowledge, UA and UB are not further metabolized by microbes, while for biosynthesized DAPs the methylation of functional hydroxy groups is common. For example, AOH is naturally produced as a mixture with AME, probably increasing its bioavailability and potentially its adverse effects [5].
3. Pharmacokinetics
Animal data on pharmacokinetics of urolithins and Alternaria toxins are only comparable to a limited extent due to differences in used species and experimental setups. However, according to a quick survey using the SwissADME quantitative structure–activity relationship (QSAR) tool [22], the bioavailability of major urolithins and fungal DAPs is predicted to be very similar (Table 1). UA and AOH, as well as UB and AME, share a comparable lipophilicity, and all four compounds have a 0.55 probability to be at least 10% bioavailable from oral uptake in rats, referred to as “bioavailability score” [23]. One exception is the blood brain barrier (BBB) permeation that is predicted only for UA/UB, not for AOH/AME (Table 1), which might be of high interest in the scope of neuroprotective effects that are proposed for UA. In line with this prediction, the presence of UA in mammalian brains was recently confirmed [24], while AOH was not reported to reach the brains of exposed mice in another study [25].
Table 1. Pharmacokinetic parameters of major natural DAPs. Shown are: octanol-water partition coefficients (PO/W), gastrointestinal (GI) adsorption, blood brain barrier (BBB) permeability and bioavailability score, as predicted by the SwissADME QSAR [21].
| log PO/W | GI Absorption | BBB Permeant | Bioavailability Score | |
| UA | 2.06 | high | yes | 0.55 |
| UB | 2.48 | high | yes | 0.55 |
| AOH | 2.17 | high | no | 0.55 |
| AME | 2.55 | high | no | 0.55 |
4. Bioactivity Profiles
To address the question of whether significant differences exist between those compounds in the impact on human health, one must ask if the additional functionalization of AOH/AME, particularly the methylation of the DAP scaffold, might serve as a driving force of toxicity.
4.1. Topoisomerase Poisoning and Genotoxicity
In two-digit micromolar concentrations, AOH and AME are well described to act genotoxic in human cancer cell models by poisoning topoisomerase (topo) II, an enzyme critical to the maintenance of DNA integrity during replication and transcription [29,30]. To a lesser extent, the induction of oxidative stress might play a role in genotoxicity as well [31]. To act as topo poison, a molecule has to stabilize the so-called “cleavable complex” between the enzyme and DNA, preventing the ligation of a previously induced gap in the phosphate backbone of the DNA, which may then persist as a strand break [32].
4.2. Endocrine Activity
AOH and AME are reported as estrogen receptor (ER) agonists, resulting in related gene transcription and a growth stimulation of ER-positive cells [37]. Moreover, several metabolites of those compounds were predicted to act estrogenic in a mixed in silico/in vitro approach [39]. AOH was also found to interact synergistically with other xenoestrogens, such as the mycotoxin zearalenone or the soy isoflavone genistein [40,41], and to exert cumulative estrogenic effects with the plasticizer bisphenol A [42] towards estrogenicity. Furthermore, the two biosynthesized DAPs were also reported to activate other steroid receptors, such as the androgen (AR) [43] and progesterone receptor [44]. Together, these findings have sparked concerns about the endocrine-disruptive potential of Alternaria toxins [5].
4.3. Inhibition of Casein Kinase 2
Casein kinase 2 (CK2) is a highly pleiotropic protein kinase whose overexpression is linked to pro-oncogenic processes [52] and anti-apoptotic effects in cancer treatment [53]. Thus, inhibition of CK2 has emerged as a therapeutic mode of action for overcoming drug resistance in cancers [54]. The DAP backbone seems to be a promising scaffold for this activity, as several representatives were predicted or reported to inhibit CK2. UA inhibited the enzyme with a IC50 of 0.39 μM and served as a precursor for the development of a much more potent inhibitor, its 4-bromo-derivative, that reached an IC50 of 0.015 nM [55]. For AOH, a similar IC50 (0.71 μM) regarding CK2 inhibition was observed in a cell-free assay, and the idea to base CK2-inhibiting drugs on its scaffold was ventilated [56]. In addition to the possible application in chemotherapy, a general chemopreventive effect of dietary CK2 inhibitors is currently discussed [57].
4.4. Mitophagy and Mitochondrial Health
Mitophagy is the cellular process of recycling damaged mitochondria that is central to mitochondrial health and of particular importance to highly stressed tissues, such as muscles [58]. It includes several pathways that can be influenced by extrinsic factors. It is well established that UA promotes mitophagy by stabilizing PTEN-induced kinase 1 (PINK1), responsible for recruiting and activating the protein Parkin, which in turn triggers the ubiquitination and thus degradation of mitochondrial proteins [59,60]. Exploiting this mechanism, the compound has even passed clinical trials as a promotor of mitochondrial and cellular health [61] and is marketed as a supplement to improve muscle health, particularly for elderly people [62].
4.5. Nrf2, Anti-Oxidative and Anti-Inflammatory Effects
In addition to the therapeutical application in the context of mitophagy, the propagation of urolithins as healthy dietary metabolites is based on their characterization as antioxidant and anti-inflammatory agents that have been extensively reviewed in recent literature [2,3]. The main mechanism behind their counteracting of oxidative stress is the activation of the Nrf2 pathway. The protein is bound to “Kelch-like ECH-associated protein 1” (Keap1) in the cytosol, which undergoes conformational changes to release Nrf2 in the presence of reactive oxygen species (ROS) or other electrophilic agents [65,66]. It then relocates into the nucleus, where it serves as a transcription factor inducing the expression of endogenous antioxidant agents and enzymes. Additionally, anti-inflammatory effects are mediated by Nrf2 via a crosstalk with NF-κB signaling [67]. Consequently, the beneficial compounds that trigger this pathway usually act slightly pro-oxidatively themselves and/or might even cause cellular oxidative stress at significantly higher concentrations.
4.6. Autophagy and Senescence
Autophagy is the process of degradation and recycling of cytosolic proteins of damaged cells, which is mostly associated with beneficial health effects, such as the prevention of cellular stress and tumor progression [79]. Several studies have reported the induction of this process after the exposure of different cells to DAPs of distinct sources. UA was found to induce this autophagy and to thereby inhibit metastasis-related biomarkers in colorectal cancer cells [80] and to protect neural cells from injury by decreasing endoplasmic reticulum (ER) stress [81]. Furthermore, there is some evidence suggesting that a part of the anti-inflammatory properties of UA might be related to increased autophagy in macrophages [82].
Interestingly, AOH was also reported to induce autophagy in macrophages, presumably via the mediation of ER stress that triggers the mTOR pathway [83]. However, in that study, a prolonged exposure of cells to AOH resulted in cellular senescence, a less desirable condition. From the two studies conducted on macrophages, it seems likely that UA and AOH exert similar effects toward the induction of autophagy in human cells.
4.7. Interactions with the Gut Microbiome
Large parts of ingested DAPs, such as AOH and AME, are excreted via the feces [26], and thus the gastrointestinal tract is probably the primarily exposed organ, which has sparked interest on potential interactions with the gut microbiome as an additional mode of action. A recent study addressed these questions and reported inhibitory effects of a complex mixture of Alternaria toxins on a multitude of bacterial strains and their ability to form biofilms [84]. However, as the extract that microbes were exposed to contained large amounts of mycotoxins with other chemical structures, a causal relationship with exposure to AOH/AME cannot be established from the published research. Likewise, experiments simulating the gastrointestinal tract revealed pomegranate extract to modulate the composition of the microbiome by increasing the prevalence of Akkermansia and Gordonibacter, particularly in the distal colon [85]. Furthermore, these changes seemed to have a direct enhancing impact on the formation of urolithins. Again, it seems difficult to draw a direct conclusion on the effects of DAPs on microbial communities due to the chemical complexity of the applied extract.
5. Conclusions
Taken together, the differentiation between “healthy” urolithins and “toxic” AOH derivatives seems to be a direct consequence of the reputation of their respective origins (superfoods vs. molds) and thus should be viewed critically. The two signature compounds of the respective groups, UA and AOH, are predicted to exert similar pharmacokinetic characteristics and share many biological activities, such as in vitro genotoxicity at high doses, the interaction with steroid receptor activation and steroid biosynthesis, the activation of the Nrf2 pathway, related anti-inflammatory effects and the induction of autophagy. However, blind spots on both sides prevent a full comparability of existing data.
On the one hand, the risk assessment of urolithins might not be fully completed yet, particularly regarding potential endocrine effects of higher doses that could hypothetically be reached via the application of pure urolithins as supplements. On the other hand—and somewhat counterintuitively—DAPs produced by Alternaria spp. and similar fungi might have a hidden potential in chemoprevention or as scaffolds for the design of therapeutic bioactives. A particularly relevant open question is whether AOH and AME would be able to promote mitophagy in a comparable way as their siblings of bacterial origin. Additionally, sub-genotoxic concentrations of mycogenic DAPs could hypothetically play a role in reducing cellular oxidative stress by activating the Nrf2 pathway, which could be utilized for the design of novel Nrf2-activating agents, e.g., for the treatment of diabetes mellitus.
It should be said that the discrepancies around research on DAPs might serve as an example of how research focus and chosen methodology can shape scientific understanding. A direct comparison of results for the two DAP classes is very challenging due to the different scientific fields (pharmacology vs. toxicology) and the respectively chosen experimentation. Nevertheless, such a comparative approach could allow for a prediction of possible biological targets for compounds of the other DAP class and thus could prove highly valuable in fueling corresponding research. In this light, it seems obvious that studies that test DAPs of different origin with a harmonized methodology would be of great value in evaluating the remaining toxicological or pharmacological questions.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222313063
This entry is offline, you can click here to edit this entry!
