Hepatic artery infusion chemotherapy (HAIC) is a well-established and common treatment for advanced hepatocellular carcinoma (HCC), particularly in East Asia. However, HAIC is not recognized internationally. Although several trials have demonstrated the safety and efficacy of HAIC, evidence corroborating its overall survival (OS) benefits compared with standard treatments is insufficient. Nevertheless, HAIC may provide prominent benefits in selected patients such as patients with portal vein thrombosis or high intrahepatic tumor burden. Moreover, HAIC has been combined with several therapeutic agents and modalities, including interferon-alpha, multikinase inhibitors, radiation therapy, and immunotherapy, to augment its treatment efficacy. Most of these combinations appeared to increase overall response rates compared with HAIC alone, but results regarding OS are inconclusive. Two prospective randomized controlled trials comparing HAIC plus sorafenib with sorafenib alone have reported conflicting results, necessitating further research. As immunotherapy-based combinations became the mainstream treatments for advanced HCC, HAIC plus immunotherapy-based treatments also showed encouraging preliminary results. The trials of HAIC were heterogeneous in terms of patient selection, chemotherapy regimens and doses, HAIC combination agent selections, and HAIC technical protocols. These heterogeneities may contribute to differences in treatment efficacy, thus increasing the difficulty of interpreting trial results.
1. Introduction
Hepatic artery infusion chemotherapy (HAIC) is a treatment modality for advanced hepatocellular carcinoma (HCC). HAIC entails infusing chemotherapeutic agents directly into hepatic tumors through the percutaneous catheterization of feeding arteries. Because HCC tumors are primarily supplied by the hepatic arteries, HAIC provides a higher intratumoral concentration of chemotherapeutic agents and avoids the first-pass effect, theoretically yielding greater treatment efficacy and less hepatocellular injury [
1]. These chemotherapeutic agents subsequently went through the body by circulation and also offered systemic anti-tumor effect but with less concentration advantage. Therefore, HAIC is basically a systemic treatment with more prominent locoregional efficacy. These peculiar features make HAIC distinct from other transarterial therapeutic approaches for HCC, such as transarterial chemoembolization (TACE) and selective internal radiation therapy (SIRT), which yield locoregional efficacy only and failed to provide survival benefit for patients with advanced HCC [
2,
3,
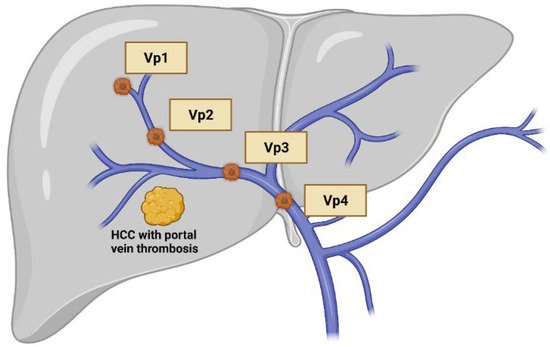
4]. Furthermore, TACE is considered as relative contraindicated in patients with portal vein thrombosis (PVT), since reduced blood supply in both portal vein system and hepatic arteries may cause substantial hepatocyte injury, especially for Vp3/4 thrombosis (
Figure 1). In contrast, HAIC can be performed safely in these patients.
Figure 1. Classification of macrovascular invasion of hepatocellular carcinoma, including portal vein thrombosis and/or tumor invasion. Vp1: the third order branch or portal vein; Vp2: the second order branch of portal vein; Vp3: the first order branch of portal vein; Vp4: the main trunk of portal vein. Created with BioRender.com.
2. HAIC Monotherapy
HAIC has long been reported as a potential therapy for advanced HCC [
12]. Before the advent of sorafenib, advanced HCC was often most effectively treated with supportive care, antiangiogenesis agents such as thalidomide [
13], or chemotherapy. These treatments conferred limited objective response rates (ORR), ranging from 0% to 21%, and were associated with a risk of high rates of hematological toxicity [
13,
14,
15,
16]. By contrast, HAIC conferred higher ORRs, ranging from 5% to 71% (
Table 1), and lower systemic toxicity [
1]. A nationwide registry study in Japan compared HAIC treatment with no active treatment for patients with advanced HCC; the study revealed that HAIC was associated with improved overall survival (OS) compared with the most effective supportive care (median survival, 14.0 vs. 5.0 months; hazard ratio [HR], 0.48;
p < 0.001) [
17]. Other retrospective studies have also reported higher efficacy of HAIC compared with transcatheter arterial chemoembolization (TACE) or systemic chemotherapy for advanced HCC [
18,
19].
Table 1. Selected studies on HAIC versus sorafenib as the first-line treatment for advanced HCC.
As a result of the SHARP clinical trial [
20] and associated Asia-Pacific trials [
21], sorafenib became the first standard systemic treatment with improved OS for advanced HCC compared with placebos. Several small-scale studies have subsequently investigated whether HAIC can yield superior benefits over sorafenib for patients with advanced HCC. Such studies have generally reported that HAIC demonstrated higher ORRs than sorafenib did, but they could not draw definite conclusions regarding OS (
Table 1) [
22,
23,
24,
25,
26,
27,
28]. In the prospective SCOOP-2 Phase 2 trial comparing HAIC with sorafenib, HAIC was even associated with a numerically shorter OS compared with sorafenib (median survival, 10.0 vs. 15.7 months,
p = 0.78). Additionally, HAIC antitumor effects on extrahepatic spread (EHS) were not specifically reported, but it was considered theoretically attenuated. Thus, HAIC monotherapy lacks sufficient evidence as a standard first-line therapy for advanced HCC.
Regarding second-line treatments and beyond, HAIC has not been directly compared with other second-line systemic therapeutic agents such as regorafenib, cabozantinib, and ramucirumab. HAIC after failure of sorafenib or other first-line treatments was reported to be effective and well tolerated, with a remarkable ORRs of approximately 30%, even in patients unsuitable for regorafenib treatment [
29,
30,
31].
Selected patient populations may, however, gain greater benefit from HAIC. Many investigators have administered HAIC to patients with macrovascular invasion (MVI), a subgroup with inferior prognosis and required prompt treatment response. Retrospective studies focusing on patients with PVT have revealed that patients receiving HAIC had a longer OS compared with those receiving sorafenib treatment [
22,
28]. HAIC also provided survival benefits for large HCC as shown in retrospective studies [
32,
33], and also in a randomized Phase 3 study comparing HAIC and TACE in large (>7 cm) intermediate HCC [
34]. Adverse events of HAIC in these studies were relatively low [
32,
34]. At the 2021 American Society of Clinical Oncology conference, Lyu et al. presented the results of FOHAIC trial comparing first-line HAIC with sorafenib in advanced HCC mainly with MVI and high tumor burden; they reported, for the first time in a prospective Phase 3 study, that HAIC could lead to a longer OS than sorafenib could (median survival, 13.9 vs. 8.2 months,
p < 0.001) [
35]. These study results support the efficacy of HAIC in patients with MVI or with large intrahepatic tumor burden.
Another area for HAIC monotherapy is in patients with poor liver function reserve, such as those with Child–Pugh (CP) Class B or C cirrhosis [
6]. For such patients, systemic treatment choice is still very limited because most therapeutic modalities for advanced HCC were developed for patients with adequate liver function. The CP-B cohort in the CheckMate-040 trial [
36] exhibited an attenuated ORR (10%) for nivolumab monotherapy, which was only half that observed for the CP-A cohort. Two retrospective studies have revealed survival benefits of HAIC over sorafenib treatment for CP-A and selected CP-B group [
26,
37], although such benefits were not consistently observed in other retrospective studies [
28,
38]. Terashima et al. [
39] published a notable retrospective study of patients receiving sorafenib or HAIC and discovered that more patients receiving HAIC exhibited sustained or improved liver function after four weeks of treatment compared with patients receiving sorafenib (72% vs. 50%,
p = 0.006). This result further indicates that HAIC may minimize injury to normal hepatocytes and possibly improves liver function by reducing tumor burden. Correspondingly, Liu et al. [
40] reported a patient of advanced HCC with CP-C who received HAIC treatment. The patient had a good partial response and his liver function reserve also improved to CP-A gradually. Therefore, HAIC may be considered as a potential first-line treatment for patients withpoor liver function reserve.
3. HAIC-Based Combination Therapy
The following characteristics of HAIC render it a suitable candidate for combination with other antineoplastic agents for advanced HCC: it is associated with fewer systemic adverse events compared with intravenous chemotherapy, and its cytotoxic mechanism is distinct from those of other HCC therapeutic modalities. Several studies have explored potential HAIC-based combination strategies (Table 2).
Table 2. Selected studies on HAIC combinations as first-line treatment for advanced HCC.
3.1. HAIC Plus Subcutaneous Interferon-Alpha
Subcutaneous or intramuscular interferon-alpha (IFN-α) has been used in combination with intravenous chemotherapy for advanced HCC to enhance antitumor activity [
55]. Subcutaneous IFN-α has also been combined with HAIC, resulting in higher ORRs than those achieved with HAIC alone, although the survival benefit of this combination is inconclusive [
42,
56,
57]. However, a randomized Phase 2 trial comparing HAIC with or without IFN-α showed inferior OS for the group treated with the HAIC–IFN-α combination [
43]. Because of such inconsistencies between study findings, IFN-α has not been routinely used in combination with HAIC.
3.2. HAIC Plus Multikinase Inhibitors
HAIC has been combined with sorafenib to leverage the synergistic effects of the combination. A randomized Phase 2 trial was conducted to compare HAIC plus sorafenib with sorafenib alone as a first-line therapy for patients with CP score of up to B7; the trial demonstrated that HAIC plus sorafenib resulted in a higher ORR (21.7% vs. 7.3%) and longer OS (median survival, 10.6 vs. 8.6 months,
p = 0.031) [
44]. Subsequently, Kudo et al. [
45] conducted the SILIUS trial, a multicenter randomized Phase 3 trial comparing frontline use of sorafenib with or without HAIC, and confirmed a higher ORR and longer time to progression (TTP) in the combination group, but the OS were similar between two groups. They also conducted a subgroup analysis and revealed the combination therapy yielded longer OS than sorafenib treatment did in patients with Vp4 PVT. He et al. [
47] reported another randomized Phase 3 trial comparing sorafenib with or without HAIC in 2019 in patients with PVT (Vp4: 37%); the results showed that patients treated with the combination therapy exhibited more favorable outcomes, including higher ORRs and longer OS periods (median survival, 13.4 vs. 7.1 months; HR 0.35;
p < 0.01). Although these two studies have reported opposite results regarding the effects of first-line HAIC combination, they differed in several aspects. First, they enrolled different patients: all patients enrolled in the study by He et al. had PVT, whereas only 63.2% of those in the study by Kudo et al. had PVT. Hepatitis B virus–related HCC was less prevalent in the study by Kudo et al. (23.4%) than in the study by He et al. (80%). Second, He et al. administered an oxaliplatin-based regimen, modified FOLFOX6, every 3 weeks, which is also a common intravenous chemotherapy regimen for advanced HCC in China; by contrast, the regimen in the SILIUS trial was cisplatin plus 5-fluorouracil (5-FU) every 4 weeks. Because of inherent differences between oxaliplatin and cisplatin, the use of these two platinum-based chemotherapeutic modalities may result in different synergistic effects with sorafenib [
58]. Third, He et al. used repeated intra-arterial catheterization, which allows for the adjustment of the microcatheter tip position and the re-embolization of newly developed gastroduodenal collateral arteries. These differences may contribute to the different OS results in these two trials. In summary, HAIC combined with sorafenib could provide favorable ORR and may provide OS benefits. Further research should be conducted to explore the optimal chemotherapeutic agents, protocol procedures, and target patient populations.
Data regarding the combination of HAIC with lenvatinib are limited. A retrospective study of 24 patients treated with HAIC plus standard-dose lenvatinib reported an encouraging ORR of 58% and a disease control rate of 79% [
48]. Additional prospective studies of the combination of HAIC and lenvatinib are ongoing.
3.3. HAIC Plus Radiation Therapy
HAIC combined with radiation therapy (RT) has also been extensively investigated, particularly in subgroups of patients with PVT. Han et al. [
51] conducted a small-scale single-arm pilot study of three-dimensional conformal RT followed by HAIC for HCC; they observed an ORR of 45% with manageable adverse events. Investigators from Hiroshima University, Japan, have published a series of retrospective studies comparing HAIC plus RT with HAIC alone, focusing on patients with PVT. Their results revealed impressive ORRs in the HAIC-RT combination arm, but no significant survival benefits were observed [
52,
53]. Furthermore, Kodama et al. [
54] retrospectively reviewed the effects of HAIC plus RT compared with treatment with sorafenib in patients with major PVT (Vp3/4) by using case–control matching analysis. The HAIC-RT combination group demonstrated more favorable clinical outcomes, including OS (median survival, 9.9 vs. 5.3 months,
p = 0.002) and progression-free survival (median survival, 3.9 vs. 2.1 months,
p = 0.048). The findings of these studies indicate that HAIC plus RT may yield favorable ORRs and survival benefits; nevertheless, evidence from prospective randomized controlled studies is still unavailable.
3.4. HAIC Plus Immunotherapy
Immune checkpoint inhibitor–based combinations have changed the treatment paradigm for advanced HCC [
59,
60] and are likely to remain the cornerstone of systemic treatment in the next few years. The IMbrave150 trial compared treatment with atezolizumab plus bevacizumab and treatment with sorafenib; they reported an impressive ORR of 30% and an unprecedented OS benefit for the combination treatment over sorafenib (median survival, 19.2 vs. 13.4 months, HR 0.66) [
60,
61]. Several ongoing Phase 3 trials testing immune checkpoint inhibitors in combinations with other immuno-oncology agents or multikinase inhibitors (MKIs) are ongoing.
Chemotherapeutic modalities have been proved to be synergistic with anti-PD1/PD-L1 antibodies in several cancers, such as those of the lung and breast [
62,
63]. HAIC may also induce substantial local immune modulation in the intrahepatic tumor microenvironment of HCC. Whether HAIC plus PD1/PD-L1 blockade would have synergistic effects warrants further investigations. Preliminary results of early phase trials of PD-1 blockade plus MKIs have been promising [
59], and investigations of triplet therapy, namely anti-PD-1, MKIs, and HAIC, are ongoing. Gu et al. [
49] reported a single-center experience for six patients who received HAIC combined with apatinib and toripalimab as the first-line treatment for advanced HCC. All six patients responded to treatment (ORR, 100%), and three of the patients (50%) exhibited complete responses. He et al. [
50] presented a retrospective study in which 71 patients underwent treatment involving a combination of HAIC, lenvatinib, and toripalimab; they reported a high ORR (59%) after treatment. These encouraging results support further research on HAIC combined with other immune-based therapeutic agents.
In summary, many studies have shown positive signs for HAIC combination treatments. In particular, for patients with major PVT, HAIC plus sorafenib provided a longer OS [
45,
47]. Regarding the combination of HAIC with other therapeutic modalities, HAIC plus RT or PD-1/PD-L1 blockade also demonstrated promising results [
49,
50,
52,
53,
54]. It's believed that these HAIC-based combination treatments will become the dominant trend in clinical practice and clinical trials.
For more detailed information, please refer to:
Int J Mol Sci. 2021 Nov 28;22(23):12880. doi: 10.3390/ijms222312880.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222312880