N,N-dimethylacrylamide produces hydrogel when polymerized with cross-linkers. Moreover, poly(N,N-dimethylacrylamide) has gotten a lot of attention as it is commonly used as the hydrophilic side of copolymers due to its unique properties and high water solubility. In addition, van der Waals interactions between N,N-dimethylacrylamide and dye molecules even more increase the applicability of DMAA hydrogels.
- N
- N-dimethylacrylamide
- hydrogel
- heavy metal ions sorption
- self-healing
- adsorption techniques
1. Introduction
DMAA is an easily polymerized, nonionic monomer. The high reactivity and low initiation temperature of DMAA make it appropriate for copolymerization. Copolymers of DMAA with N,N-dimethyl-N,N-diallylammonium chloride (DMDAAC) can be used as polymeric flocculant for water treatment [1,2,3]. The unique structure of DMAA shows amelioration in hydrogels for specific application. DMAA along with other hydrogels has a three-dimensional network structure that is capable of retaining water. In addition, DMAA hydrogel can be applied for the removal of toxic metal ions from wastewater because of characteristic properties such as chemical stability and high adsorption capacity. DMAA can also be used for flocculation due to its ability to augment the molecular weight of the copolymers.
The polymer bridging mechanism is the main flocculation mechanism. Longer polymer chains can come in contact with more colloidal particles in suspension that makes longer bridges that were built among particles, which subsequently aggregate into large flocs. The bridging effect depends on the structure of polymers. Having no intermolecular hydrogen bond, DMAA has a higher possibility of binding colloidal particles that will enhance the bridging effect. That enhanced bridging effect plays an important role in flocculation. Thus, grafted polymers of DMAA have a higher flocculation effect than polyacrylamide-grafted ones [4,5,6,7,8]. The amide group of the DMAA polymer also increases the flocculation of humic acid at very low concentration [9].
Free-radical initiated polymerization of DMAA, with a cross-linker, is frequently used for DMAA-based hydrogel preparation. Initiation is most often carried out by free-radical polymerization methods. Usually, the solution polymerization of DMAA is carried out with a water-soluble cross-linker, such as methylene bis-acrylamide (MBA). Different studies of DMAA hydrogels use cross-linkers from 0.7% to 3.2% (of total monomer weight) and initiators in the range of 0.3–1.0% in different applications. For the initiation of DMAA polymers, either ammonium or potassium persulfate are used in water media [10,11,12].
Metal ions are significant in various biochemical processes for living organisms to balance the function of biological systems. For example, zinc is both crucial for the development of the immune system and against viral infections. According to recent studies, consuming 40 mg of zinc per day could be helpful against SARS-CoV-2 infection. Trace metals (zinc, selenium, copper, magnesium) maintain immune system cells, and their deficiency can make people more vulnerable to infectious infections [13,14]. Calcium in the food can accrue the benefits of vitamin D and lowers the risk of breast cancer. On the other hand, bone diseases can be caused by a lack of calcium or deficiency of vitamin D. Although calcium can lower the risk of a variety of illnesses, it can simultaneously increase the risk of acute gastrointestinal events, kidney stones, and cardiovascular disorders including myocardial infarction and stroke [15]. Iron is the key element in the hemoglobin, playing an important role for oxygen transport [16,17]. Clinical trials have shown that copper can minimize the bacterial and viral infections, while it can also be useful in preventing the spread of infectious illnesses [14,18]. However, the presence of an excessive amount of metal ions has harmful effects on human health and the environment [19,20,21,22].
2. N,N-Dimethylacrylamide-Based Hydrogels for Dye Removal
Many dyes and organic molecules are common cationic hazardous chemicals that must be eliminated in a wastewater treatment facility. Removing dyes from wastewater is very important due to their high toxicity for human health. If the dyes enter into the human body, they can produce bioaccumulation and augment carcinogenicity. Organic dyes not only cause damage to human health but also reduce the photosynthesis by preventing the penetration of light through water, which can negatively impact on the quality of water bodies [27,28,29,30]. Recently, scientists have developed many methods to remove dyes from water. One of the prominent ways for eliminating dyes from wastewater is hydrogel techniques. There are many hydrogels that have been used for adsorbing dyes from water such as N,N-dimethylacrylamide (DMAA).
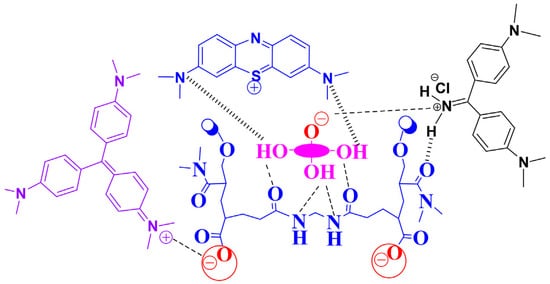
N,N-dimethylacrylamide produces hydrogel when polymerized with cross-linkers. Moreover, poly(N,N-dimethylacrylamide) has gotten a lot of attention as it is commonly used as the hydrophilic side of copolymers due to its unique properties and high water solubility. In addition, van der Waals interactions between N,N-dimethylacrylamide and dye molecules even more increase the applicability of DMAA hydrogels. Hossain et al. proved this theory when they synthesized DMAA-based 3-methacryloxypropyltrimethoxysilane hydrogels and used them for removing methylene blue cationic dye from waste water. The maximum adsorption value of the DMAA-based hydrogel was 131.58 mg/g [31]. Since DMAA acts as a hydrogen bond acceptor, it could help to enhance the material’s adsorption properties. Apart from hydrogen bonding, the dipole–dipole interactions of amide groups of the DMAA hydrogels can also play a big part on the adsorption of methylene blue and toluidine blue dyes. Preetha and Vishalakshi synthesized Karaya gum-grafted-poly(N,N-dimethylacrylamide) hydrogel and studied the sorption of four different cationic dyes: methylene blue, crystal violet, rhodamine, and toluidine blue on the hydrogels at different pH levels. The findings of this study proved that the adsorption capacity of DMAA-based hydrogel was very high compared to many other adsorption materials [32]. The adsorption capacity of DMAA hydrogels augments with the increasing of pH of the solution due to the hydrolysis and protonation. Mechanisms of adsorption have been noticed on the works of Hossain et al. ( Figure 1 ) [31,33]. At low value of pH, dimethyl acrylamide groups are protonated, and a positively charged NH(CH 3) 2+ surface leads to electrostatic interaction with the negatively charged dye molecules, thus improving the negatively charged dye removal [34,35].

The synthesized DMAA hydrogels were seen to be extremely effective in removing cationic dyes due to electrostatic interaction and hydrogen bonding in the DMAA backbone. The formation of hydrogen bonding between –NH- groups in DMAA with the electronegative N in dye molecules as well as electrostatic attractions between cationic groups of dye molecules and carbonyl groups were the main driving force behind its attraction. The mechanisms of interactions are presented in Figure 2 [36,37].
The amounts of adsorbed dye per unit mass of hydrogel at any time t (Q t , mg ∗ g −1 ) and at equilibrium (Q e , mg ∗ g −1 ) are calculated by using the following equations: (1) Q t ( mg * g − 1) = ( C 0− C t ) * V ( L ) m ( g ) (2) Q e ( mg * g − 1) = ( C 0− C e ) * V ( L ) m ( g ) where C 0 and C e are the initial and equilibrium dye concentrations (mg L −1 ), respectively, C t is the dye concentration at time t, V is the volume of the solution added (L), and m is the amount of hydrogel (g).
3. N,N-Dimethylacrylamide Hydrogels for Self-Healing Materials
Self-healing hydrogels are a form of polymer hydrogel that has the ability to heal itself. Self-healing is the formation of new bonds when old bonds are broken within a material. Electrostatic attraction forces between molecules can create the formation of new bonds by reconstructive covalent side chain or non-covalent hydrogen bonding. These properties of self-healing hydrogel have attracted attention in many fields. Hydrogels with self-healing, injectable, and stimuli-responsive properties can have many advantages. For example, self-healing hydrogels implanted in the body can form their original state after damaging by external forces. This increases the body’s safety and reduces the economic cost [51]. On the other hand, self-healing hydrogels easily release and carry biological active compounds [52].
The hydrogen bond and electrostatic attraction forces between N,N-dimethylacrylamide molecules can hold and easily release bioactive compounds (drugs) depending on the medium of the body. Du et al. prepared N,N-dimethylacrylamide-stat-3-acrylamidophenylboronicacid statistical copolymers (PDMAA-stat-PAPBA) and poly(glycerolmonomethacrylate) (PGMA) chains grafted cellulose nanocrystals (CNC-g-PGMA) and studied the mechanical and self-healing properties of the new hydrogel. According to the study, the self-healing properties were enhanced by adding N,N-dimethylacrylamide. Self-healing N ,N-dimethylacrylamide hydrogels are not only drug carrier but also can be used in biosensors. Hou et al. developed N ,N-dimethylacrylamide self-healing hydrogels with thermo-responsive properties that can be used in medicine as a body temperature regulator [53]. The unique structure of N,N-dimethylacrylamide hydrogels is mainly due to having both hydrophobic interactions and hydrogen bonding in its hydrogels [54]. The hydrogen bonding and hydrophobic interactions between the methyl groups of the N,N-dimethylacrylamide network are responsible for the reentrant transition conduct of the hydrogels. The hydrogels hold up to about 4200% strain, and the damage created in the gels could be healed at 50 °C within 10 h ( Figure 4 A,B) [55,56].
4. N,N-Dimethylacrylamide Hydrogels for Enhancing Mechanical Properties of the Materials
The hydrophobic interactions, hydrogen bonding, and ion–dipole interactions in the single structure of N,N-dimethylacrylamide can augment the mechanical strength of hydrogel. Weng et al. modified natural polysaccharides with DMAA and obtained highly porous hydrogels with high mechanical strength. Even though the obtained hydrogel contained more than 90% water, it still withstood high compressive strength [57,58,59]. Furthermore, in Figure 5 , hydrogels with and without the addition of DMAA were demonstrated. Despite the fact that they were both of the same toughness, the one containing DMAA was deformed by 26% without producing noticeable damage, whilst the other was broken entirely. In DMAA-containing hydrogels, the energy dissipation process inhibits force localization and therefore prevents the hydrogel matrix from macroscopic damage [60,61,62].
Pendant vinyl groups in DMAA monomer can be incorporated into other monomers as a spacer and can increase the flexibility of hydrogels [63,64,65,66]. C.B. Oral et al. included DMAA monomer into silk fibroin hydrogel and changed the brittle hydrogel into a stretchable hydrogel with an elongation ratio up to 370% [60].

This entry is adapted from the peer-reviewed paper 10.3390/gels7040234
