Anthocyanins are flavonoid pigments broadly distributed in plants with great potential to be used as food colorants due to their range of colors, innocuous nature, and positive impact on human health. However, these molecules are unstable and affected by pH changes, oxidation and high temperatures, making it very important to extract them using gentle non-thermal technologies.
- grapes
- anthocyanins
- emerging non-thermal technologies
1. Introduction
In most grape varieties, anthocyanins are located in the exocarp (skins) ( Figure 1 A,B), which are the layers of cells in the outer surface of the berry; only a few varieties also have anthocyanins in the pulp [16]. The skins have a thicker cell wall than the pulp to protect the berry mechanically and against rot and pests.
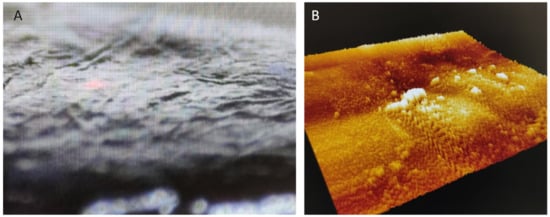
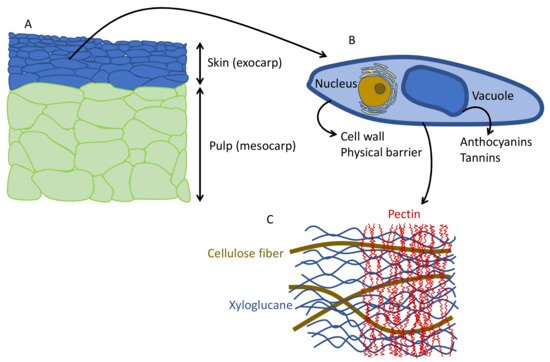
The structure and shape of the cells in the berries are flat cells in the skin and large polyhedral cells in the pulp ( Figure 2 A). Anthocyanins are located in the cells of the skin, inside the vacuole ( Figure 2 B). To extract the anthocyanins and to keep enough color (not only in red winemaking, but also in red juice production), it is necessary to disaggregate the cell wall polysaccharides, mainly the pectins ( Figure 2 C). In conventional winemaking, depolymerization of the cell wall and separation of polysaccharide fibers is achieved during maceration by means of soaking, fermentation temperature and mechanical treatments (i.e., punch downs, pump overs, délestage) [18].
In addition, cryomacerations (cold soak) by heat exchanger cooling or dry ice can be used to preferentially extract anthocyanins and aroma compounds in the absence of fermentation [19]. This is advantageous because it reduces the extraction of tannins whose solubility is lower in the absence of alcohol and better reduces astringency in young wines and juices. Another powerful technology to quickly degrade cell wall pectins and promote the extraction of anthocyanins, tannins and aroma compounds is the use of pectolytic enzymes [20], especially endo-polygalacturonases that break the pectin sequence depolymerizing the cell wall and releasing the pigments in the juice.
2. Use of High-Pressure Technologies to Extract Anthocyanins
The use of high-pressure technologies is growing exponentially in the food industry. A PubMed search using the keywords high, pressure and food yields 40,077 research articles in the period 1970–2021 with 36,920 since 2000. Although several technologies can be found, research with continuous (Ultra)-High Pressure Homogenization processes (UHPH and HPH) and discontinuous High Hydrostatic Pressure (HHP) technologies stand out. All of them share gentle food processing as they are non-thermal treatments with low impact on food quality, sensory constituents and nutraceutical components [25,38,39,40,41]. HHP and UHPH technologies are industrially implemented and several brands compete in the market. In batch technologies, the leading companies are Hiperbaric (https://www.hiperbaric.com/es/ (accessed on 2 November 2021)) and Avure (https://www.jbtc.com/es/north-america/foodtech/products-and-solutions/brands/avure-technologies (accessed on 2 November 2021)).
3. Pulsed Electric Fields (PEFs) in the Extraction of Anthocyanins
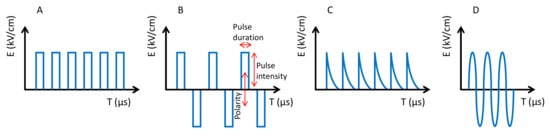
Like the previous ones (HHP and UHPH), PEFs have become a global technology with numerous applications in food processing, preservation and stabilization [67,68,69,70,71]. PEF is based on the use of high intensity electric fields (3–40 kV/cm) for a very short time (milli-micro seconds). Food is processed by PEF when it passes through two electrodes. The Electric Field Strength (E) is the voltage (kV) divided by the distance between the electrodes (cm), i.e., E = V/d. PEF systems are currently available on an industrial scale for food processing in the range of 50–10,000 L/h for fluids and 1–70 tonnes/h for solids such as French fries. The effect of PEFs is the poration of cells at the nanoscale, which affects the selective permeability [72]. These pores are difficult to observe by electronic microscopy. However, the pores produce various effects depending on size and number, tending to increase cell permeability, thus facilitating the extraction of cell compounds (e.g., anthocyanins and many others), the entry of compounds and the temporal or definitive inactivation of cells depending on the intensity [73]. Pulses can be applied in several modalities. The main parameters are the pulse shape (i.e., squared, exponential, sinusoidal), the polarity (i.e., monopolar or bipolar), the number of pulses and the pulse duration ( Figure 6 ). The intensity and effectiveness of the treatments depend on the above parameters with squared bipolar pulses being more effective and the number of pulses making the process more powerful. Even when the pulse duration also improves the efficacy, it should be kept at a low value because it affects the temperature of the food by ohmic heating.
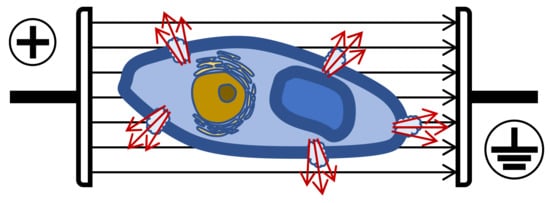
Plant cells need lower intensities than microorganisms, especially bacteria, depending on size and shape. To induce permeabilization in plant cells (size 40–200 µm), E must be 1–2 kV/cm, while in microorganisms (size 1–10 µm), 12–20 kV/cm are required [74]. Therefore, to extract bioactive molecules from vegetal tissues, less than 5 kV/cm is necessary, however, for microbial inactivation, E should normally be higher than 10 kV/cm. When plant cells are pored (i.e., grape skins), the consequence is an increased extraction of biomolecules such as anthocyanins, tannins and aroma compounds ( Figure 7 ).
At pilot and industrial scale, several works have demonstrated the efficiency of PEFs to increase the extraction of anthocyanins, and other phenols at low temperature while preserving their antioxidant capacity ( Table 1 ). Currently grapes or by-products (grape pomace) can be processed continuously at a flow rate of several hundreds to a few tonnes of kg per hour (118 kg/h, [51]; 500 kg/h [53], 1900 kg/h [52]). Usually, the crushed gape is pumped by a progressive cavity pump [51] or a peristaltic pump [52] and later processed in a collinear chamber by applying exponentially decay pulses or, more frequently, squared pulses of an electric field strength ranging from 2 to 10 kV/cm [26,28,48,49,52,53]. Anthocyanin extraction increases in the range of 17–100% ( Table 1 ) depending on processing conditions and post-maceration time. The temperature is increased by only 2–15 °C [55], therefore it is easy to work at room temperature or under refrigerated conditions. In addition to improved anthocyanin and phenol extraction, PEFs can be used for gentle non-thermal pasteurization of the must, thus improving the implantation of non- Saccharomyces starters [55] and potentially reducing the use of SO 2. The effect of PEFs on the extraction of phenolic compounds from seeds has also been reported and should be considered in winemaking processes [75,76].
Table 1. Molecular structure, substitution pattern in the B-ring (R1 and R2), acylation patterns (R3), maximum λ (nm), and color of grape’s anthocyanins.
| Anthocyanin | R1 | R2 | R3 | λ max 1 | Colour | [M]+/Aglycon (m/z)2 |
|---|---|---|---|---|---|---|
| Delphinidin-3-O-gucoside | ‒OH | ‒OH | ‒H | 526.9 | Red | 465/303 |
| Cyanidin-3-O-glucoside | ‒OH | ‒H | ‒H | 518.4 | Orange-red | 449/287 |
| Petunidin-3-O-glucoside | ‒OH | ‒OCH3 | ‒H | 528.1 | Red | 479/317 |
| Peonidin-3-O-glucoside | ‒OCH3 | ‒H | ‒H | 518.4 | Orange-red | 463/301 |
| Malvidin-3-O-glucoside | ‒OCH3 | ‒OCH3 | ‒H | 529.3 | Red | 493/331 |
| Delphinidin-3-O-(6-O-acetyl)-glucoside | ‒OH | ‒OH | ‒COCH3 | 529.3 | Red | 507/303 |
| Cyanidin-3-O-(-6-O-acetyl)-glucoside | ‒OH | ‒H | ‒COCH3 | 520.8 | Red | 491/287 |
| Petunidin-3-O-(-6-O-acetyl)-glucoside | ‒OH | ‒OCH3 | ‒COCH3 | 530.5 | Bluish-red | 521/317 |
| Peonidin-3-O-(-6-O-acetyl)-glucoside | ‒OCH3 | ‒H | ‒COCH3 | 520.8 | Red | 505/301 |
| Malvidin-3-O-(-6-O-acetyl)-glucoside | ‒OCH3 | ‒OCH3 | ‒COCH3 | 530.5 | Bluish-red | 535/331 |
| Delphinidin-3-O-(6-O-p-coumaroyl)-glucoside | ‒OH | ‒OH | ‒COCH = CHC6H4‒OH | 534.2 | Bluish-red | 611/303 |
| Cyanidin-3-O-(-6-O-p-coumaroyl)-glucoside | ‒OH | ‒H | ‒COCH = CHC6H4‒OH | 525.7 | Red | 595/287 |
| Petunidin-3-O-(-6-O-p-coumaroyl)-glucoside | ‒OH | ‒OCH3 | ‒COCH = CHC6H4‒OH | 535.4 | Bluish-red | 625/317 |
| Peonidin-3-O-(-6-O-p-coumaroyl)-glucoside | ‒OCH3 | ‒H | ‒COCH = CHC6H4‒OH | 524.5 | Red | 609/301 |
| Malvidin-3-O-(-6-O-p-coumaroyl)-glucoside | ‒OCH3 | ‒OCH3 | ‒COCH = CHC6H4‒OH | 535.4 | Bluish-red | 639/331 |
| Malvidin-3-O-(-6-O-caffeoyl)-glucoside | ‒OCH3 | ‒OCH3 | ‒COCH = CHC6H3‒(OH)2 | 536.6 | Bluish-red | 655/331 |
| Molecular structure | 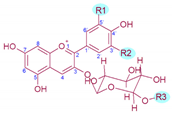 |
|||||
4. Ultrasounds (USs) in the Extraction of Anthocyanins
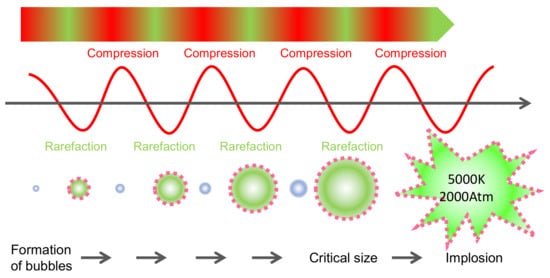
Ultrasounds (USs) are mechanic waves with a frequency above 20 kHz, which is not perceptible to the human ear (typically in the range 20 Hz–20 kHz) [77]. It is a key technology for obtaining bioactive compounds (e.g., anthocyanins), like the others described above, as it can be considered a sustainable ‘green’ extraction method [78] as it does not use organic solvents and is gentle to heat-sensitive molecules [79]. The compression and rarefaction of the products produced by the US waves produce the successive reduction in size and expansion of the bubbles formed by cavitation ( Figure 8 ). When these bubbles collapse, large amounts of energy are released, reaching localized temperatures of 5000 °K and pressures of 200 MPa [80]. These phenomena are responsible for the depolymerization of biostructures [80] and facilitate the extraction of molecules from plant tissues. Depolymerization can occur by bubble collapse, cavitation or degradation of the polymer by impact with radicals formed during sonication [81]. Depolymerization of cell wall polysaccharides accelerates the release of anthocyanins from the skin cells in grapes ( Figure 9 ). The extraction of anthocyanins in water within a few minutes and the increase in temperature due to the cavitation effect can be observed. High power ultrasounds with the best extraction potential are considered to be in the range of 20–25 kHz [78].
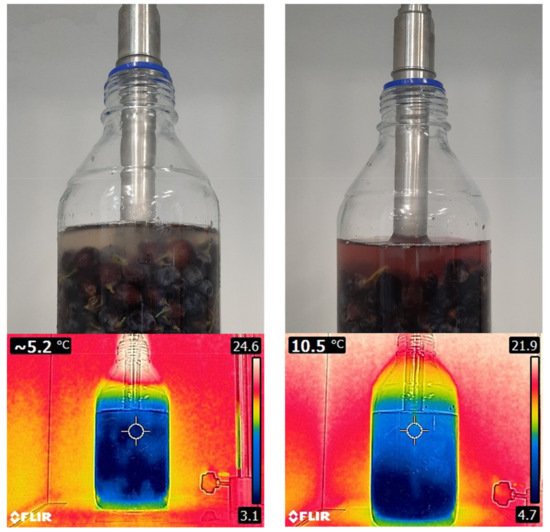
Figure 9 shows the application of USs on grape berries by means of a sonotrode and reveals, after a few minutes, how the anthocyanins are extracted to the surrounding media (water) due to the depolymerization of the cell walls of the grape skins. Additionally, the heating effect produced by cavitation can be observed, which in this case is about 5 °C in the center of the flask according to infrared thermography.
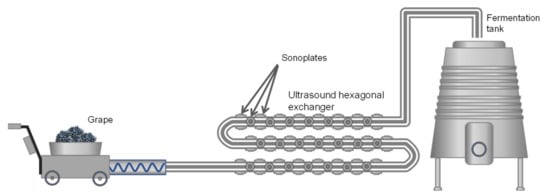
There are several systems for applying USs to plant tissues with the aim of favoring the extraction of compounds: Ultrasound baths, sonotrodes, sonoplates. However, on an industrial scale, the most effective system is the use of continuous tubular exchangers on the external surface of which sonoplates are distributed to apply US waves during the flow of the mash or liquid through the exchanger. For a better distribution of the sonoplates on the exchange surface, the section is usually hexagonal instead of circular ( Figure 10 ).
This technology has been used to process Tempranillo grapes, achieving the same anthocyanin content in a final wine after only continuous US treatment and 72 h of skin maceration as in the control wine [82]. In discontinuous treatment at the laboratory scale, the USs have been shown to increase the extraction of anthocyanins and phenols by more than 50% compared to controls [56]. US can also be applied continuously after the application of pectolytic enzymes at industrial level, increasing color intensity by 18% and total polyphenols by 21% in wines [57]. The use of US-assisted extraction can be improved by optimizing other physicochemical parameters (temperature, ethanol and time), thus reaching a maximum of 6.26 mg/mL under the best conditions of 45.14 °C, 52.3% ethanol and 24.5 min [83]. USs can be used to improve extraction and/or reduce extraction time in grapes [56,57,82], and by-products such as pomace [33] and lees [58]. USs have been applied to Vitis vinifera L. varieties Cabernet Franc [84], Tempranillo [82], Tannat [56], and Monastrell [57]. The influence of the US frequency has also been analyzed, considering the values of 12.5, 25, and 37.5 kHz, as well as from 12.5 to 25 kHz, the extraction of anthocyanins increased by 18% in grape pomace, however, the higher the frequency, the lower the extraction [59].
This entry is adapted from the peer-reviewed paper 10.3390/antiox10121863
