Photorefractive materials are capable of reversibly changing their index of refraction upon illumination. That property allows them to dynamically record holograms, which is a key function for developing an updateable holographic 3D display. The transition from inorganic photorefractive crystals to organic polymers meant that large display screens could be made.
- photorefractive
- 3D display
- holography
- energy levels
- polymer
- electro optic
- birefringence
- holographic stereogram
1. Introduction
Holograms are known first and foremost for their ability to project 3D images. Holographic 3D images were demonstrated in the early 1960s by Leith, and Upatnieks [1], as well as Denisyuk [2]. What distinguishes holography from other 3D technologies is its ability to reproduce all the human 2D and 3D visual cues: occlusion, parallax, accommodation, and vergence [3][4][5]. This makes holography a strong contender to obtain the ultimate 3D display that would be able to project images as good as the real world [6][7].
From the early work on holographic imaging, many accomplishments have been made regarding the rendering of 3D images using holograms. The depth of field has been enlarged by using long coherence lasers [8]; for example, full color reproduction has been achieved, thanks to 3 color lasers recording [9][10]; reproductions of large scenes and life subjects have been made possible by using short pulse lasers [11]; and rainbow holograms can be viewed with white light [12]. Today, it is possible to display convincing holographic reproductions of artifacts with exquisite details and in full color [13][14].
After the series of successes experienced in the field of static holographic imaging in the first half of the 1960s, there was a good hope that a holographic display presenting motion would appear soon after. Additionally, indeed, the first holographic motion picture was demonstrated by De Bitetto in 1968 and Jacobson in 1969 [15][16]. Holographic motion pictures use a succession of pre-recorded holograms that are projected fast enough to give the impression of movement.
To obtain a dynamic holographic 3D display, several approaches are possible: either using an electronically controlled phase modulator (such as a liquid crystal or acousto-optic modulator) [17][18][19][20], or using a refreshable holographic recording material, such as photorefractive polymer. The present review article focuses on this latter case, specifically the development of photorefractive materials and their use for holographic 3D display.
2. Overview of the Photorefractive Effect
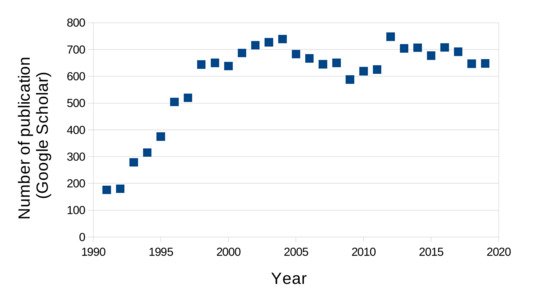
The photorefractive effect is defined as the reversible change of a material index of refraction upon illumination. Initially observed in inorganic crystals [21], it was then discovered in organic materials [22] and then polymers [23]. Since this original publication, the number of papers mentioning photorefractive polymer has grown over the years to stabilize at around 700 per year. Figure 1 shows this trend obtained from the Google Scholar search website, which indicates that the topic of photorefractive polymers is indeed a very active field of research.
To distinguish the photorefractive effect from other photo-induced index change mechanisms, such as photochromism, photobleaching, or molecular hole burning, the photorefractive effect was specified by its mode of action that involves charge photo-initiation (such as in photovoltaic material), local redistribution of the said charges into a local space-charge field, and, ultimately, the change of the refractive index due to non-linear Pockels effect or molecular reorientation [24].
This seemingly simple effect: a light-induced reversible refractive index change gives rise to a plethora of macroscopic observations and applications, such as self-focusing [25], beam fanning [26], two beam coupling [27], four wave mixing [28], holographic data storage [29], image processing [30], image through scattering media [31], and refreshable holographic recording [32]. For an extended review of the different applications of photorefractive materials, see, for example, References [33][34].
Compared to other holographic recording materials, such as dichromated gelatin or silver halide, that need post-processing for the hologram to appear, or to photopolymers that record permanent holograms, photorefractive materials represent an interesting platform for the development of an updateable 3D display. However, to do so requires several specific characteristics: the material should be sensitive enough so that a reasonable amount of power can be used to write the hologram [35][36], the material should be efficient so that high contrast hologram can be recorded [37][38], the material should be highly dynamic so thata fast refresh rate can be achieved [39][40], the material should be transparent to red, green, and blue light so that color holograms can be displayed [41][42][43], the material should withstand a high electric field without breaking down [44][45], the material should be processed in large film to achieve large screens that display large holograms [46][47], and the material should have a long phase stability so that the display can be used for an extended period of time [48][49].
3. Photorefractive Effect in Polymers
Polymer materials can demonstrate photorefractive behavior, thanks to a cascade of events that is important to understand clearly in order to optimize the different figures of merits. Different species of molecules are responsible for each of these events. In the following list, we introduce the different phenomena that led to the photorefractive effect, in their order of appearance, together with the molecular species responsible for it to happen: Charge photo-generation ↔ sensitizer molecules. Charge transport ↔ polymer chains. Charge trapping ↔ conformational traps. Space-charge field ↔ electron and hole differential relocation. Index modulation ↔ chromophore molecules.
In polymer material, the detailed analysis of the molecular energy levels has led to a better understanding of the charge transport mechanism and space charge field formation [50]. Most notably, the comparison between the time constant of polyvinylcarbazole (PVK)-based material versus poly(acrylic tetraphenyldiaminobiphenyl) (PATPD)-based polymer showed that deep charge trapping can slow down the response time of the material [44]. In Figure 2 , the energy levels of PVK and PATPD-based photorefractive materials are presented. On the left panel, it can be seen that, because the HOMO of the PVK polymer is relatively low (5.92 eV), deep traps are present in the form of chromophores 7-DCST (4-homopiperidinobenzylidenemalononitrile) and DBDC (3-(N,N-di-n-butylaniline-4-yl)-1-dicyanomethylidene-2cyclohexene) molecules with levels at 5.90 eV and 5.62 eV, respectively. These deep traps attract the charges, slowing down their transportation along the polymer manifold. The PVK-based photorefractive material has a response time measured in the hundreds of milliseconds. On the right panel, the PATPD HOMO level is too high (5.43 eV) for the holes to be trapped into the 7-DCST or DBDC molecular levels, allowing for a faster charge transport and a photorefractive effect with a response time measured in the tens of milliseconds.
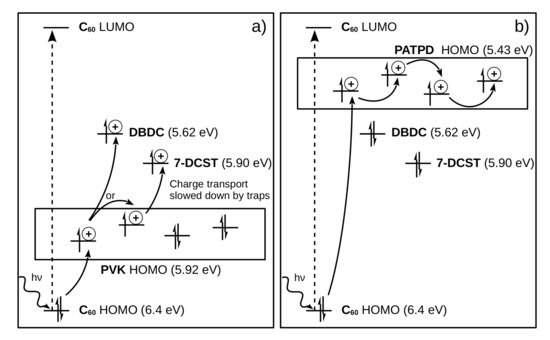
Most recently, it also has been demonstrated that, by reducing the dispersion of the energy levels in the polymer manifold, a faster charge transport can be accomplished. This happens because the charge is not trapped in local maximum but is, rather, transferred from molecule to molecule without loss of momentum [51]. In addition, the role of the plasticizer as trapping site has been reported to achieve very high refresh rate and diffraction efficiency [52].
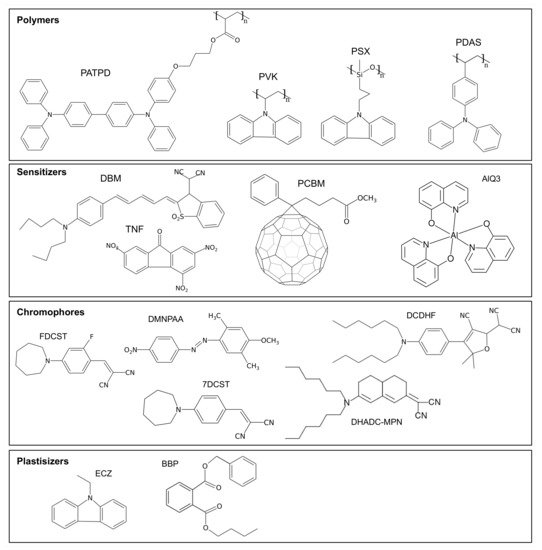
The polymer matrix used in photorefractive compounds is not only responsible for the structural integrity of the material but is also used to support charge transport. While a large array of photoconductive polymers has been used over time, the two most predominant matrices found in the literature are PVK and PATPD (see Figure 3 for their chemical structure). Both of these materials are hole conductors, meaning that the excited electrons stay in place in the excited LUMO level of the sensitizer, and it is the holes that are transported over the polymer chain by a hopping mechanism.
4. Photorefractive-Based 3D Display
The unique feature of photorefractive materials over any other dynamic holographic recording mechanism, such as molecular hole burning, molecular reorientation, or photobleaching, is that the sensitivity of photorefractive media can be improved by increasing the external applied voltage. The voltage and, thus, the efficiency is only limited by the dielectric breakdown value ( E m a x ) at which the material experiences a catastrophic failure.
The dynamic capability of photorefractive materials is often demonstrated by recording the changing images displayed on a spatial light modulator (SLM) [52][53]. However, the image coming from these SLMs is only 2D. Therefore, the hologram recorded in the holographic material using SLMs is also 2D and lacks depth information. To display 3D images, another approach needs to be taken, one of which is holographic stereograms.
Even used with permanent recording material, holographic stereogram printing technique is the subject of intense research to improve resolution, depth of field, field of view, color gamut, and other aspects of the 3D image [54][55][56].
To add a third color and produce a full red, green, and blue gamut, a third set of recording beams can be added without causing unwanted interference by using a polarization orthogonal to the other sets of beams. Using p-polarized recording beams slightly reduces the interference pattern contrast, but this could be compensated by using that polarization to record the color that is the most efficiently transmitted (usually red).
This entry is adapted from the peer-reviewed paper 10.3390/ma14195799
References
- Leith, E.N.; Upatnieks, J. New techniques in wavefront reconstruction. J. Opt. Soc. Am 1961, 51, 1469–1473.
- Denisyuk, Y.N. On the reflection of optical properties of an object in a wave field of light scattered by it. Dokl. Akad. Nauk SSSR 1962, 144, 1275–1278.
- Dunn, D.; Tursun, O.; Yu, H.; Didyk, P.; Myszkowski, K.; Fuchs, H. Stimulating the Human Visual System Beyond Real World Performance in Future Augmented Reality Displays. In Proceedings of the 2020 IEEE International Symposium on Mixed and Augmented Reality (ISMAR), Porto de Galinhas, Brazil, 9–13 November 2020; pp. 90–100.
- Howard, I.P. Perceiving in Depth, Volume 1: Basic Mechanisms; Oxford University Press: Oxford, UK, 2012.
- Park, M.C.; Mun, S. Overview of measurement methods for factors affecting the human visual system in 3D displays. J. Disp. Technol. 2015, 11, 877–888.
- Blanche, P.A. Toward the Ultimate 3-D Display. Inf. Disp. 2012, 28, 32–37.
- Matsushima, K. Introduction to Computer Holography: Creating Computer-Generated Holograms as the Ultimate 3D Image; Springer Nature: Basingstoke, UK, 2020.
- Bjelkhagen, H.; Brotherton-Ratcliffe, D. Ultra-Realistic Imaging: Advanced Techniques in Analogue and Digital Colour Holography; CRC Press: Boca Raton, FL, USA, 2013.
- Upatnieks, J.; Marks, J.; Fedorowicz, R. Color holograms for white light reconstruction. Appl. Phys. Lett. 1966, 8, 286–287.
- Bjelkhagen, H. Colour holography: The ultimate 3D imaging technique. Imaging Sci. J. 2011, 59, 83–89.
- Siebert, L. Large-scene front-lighted hologram of a human subject. Proc. IEEE 1968, 56, 1242–1243.
- Benton, S.A. Hologram reconstruction with extended incoherent sources. J. Opt. Soc. Am. 1969, 59, 1545.
- Sarakinos, A.; Lembessis, A. Color Holography for the Documentation and Dissemination of Cultural Heritage: OptoClonesTM from Four Museums in Two Countries. J. Imaging 2019, 5, 59.
- Gentet, P.; Gentet, Y.; Choi, P.H.; Lee, S.H. Evaluation of the realism of a full-color reflection H2 analog hologram recorded on ultra-fine-grain silver-halide material. Open Phys. 2019, 17, 449–457.
- De Bitetto, D. A holographic motion picture film with constant velocity transport. Appl. Phys. Lett. 1968, 12, 295–297.
- Jacobson, A.; Evtuhov, V.; Neeland, J. Motion picture holography. Appl. Phys. Lett. 1969, 14, 120–122.
- Bove, V.M., Jr. Holographic television. In Optical Holography; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–82.
- Slinger, C.; Cameron, C.; Stanley, M. Computer-generated holography as a generic display technology. Computer 2005, 38, 46–53.
- An, J.; Won, K.; Kim, Y.; Hong, J.Y.; Kim, H.; Kim, Y.; Song, H.; Choi, C.; Kim, Y.; Seo, J.; et al. Slim-panel holographic video display. Nat. Commun. 2020, 11, 1–7.
- Jolly, S.; Savidis, N.; Datta, B.; Smalley, D.; Bove, V.M., Jr. Progress in transparent flat-panel holographic displays enabled by guided-wave acousto-optics. In Practical Holography XXXII: Displays, Materials, and Applications; International Society for Optics and Photonics: Bellingham, DC, USA, 2018; Volume 10558, p. 105580L.
- Ashkin, A.; Boyd, G.; Dziedzic, J.I.; Smith, R.; Ballman, A.; Levinstein, J.; Nassau, K. Optically-induced refractive index inhomogeneities in LiNbO3 and LiTaO3. Appl. Phys. Lett. 1966, 9, 72–74.
- Sutter, K.; Hulliger, J.; Günter, P. Photorefractive effects observed in the organic crystal 2-cyclooctylamino-5-nitropyridine doped with 7, 7, 8, 8-tetracyanoquinodimethane. Solid State Commun. 1990, 74, 867–870.
- Ducharme, S.P.; Scott, J.; Twieg, R.; Moerner, W. Observation of the photorefractive effect in a polymer. Phys. Rev. Lett. 1991, 66, 1846.
- Chen, F. A Laser-Induced Inhomogeneity of Refractive Indices in KTN. J. Appl. Phys. 1967, 38, 3418–3420.
- Christodoulides, D.N.; Coskun, T.H.; Mitchell, M.; Segev, M. Theory of incoherent self-focusing in biased photorefractive media. Phys. Rev. Lett. 1997, 78, 646.
- Feinberg, J. Asymmetric self-defocusing of an optical beam from the photorefractive effect. JOSA 1982, 72, 46–51.
- Walsh, C.; Moerner, W. Two-beam coupling measurements of grating phase in a photorefractive polymer. JOSA B 1992, 9, 1642–1647.
- Cronin-Golomb, M.; Fischer, B.; White, J.; Yariv, A. Theory and applications of four-wave mixing in photorefractive media. IEEE J. Quantum Electron. 1984, 20, 12–30.
- Poga, C.; Lundquist, P.; Lee, V.; Shelby, R.; Twieg, R.; Burland, D. Polysiloxane-based photorefractive polymers for digital holographic data storage. Appl. Phys. Lett. 1996, 69, 1047–1049.
- Kippelen, B.; Peyghambarian, N.; Lyon, S.; Padias, A.; Hall, H. New highly efficient photorefractive polymer composite for optical-storage and image-processing applications. Electron. Lett. 1993, 29, 1873–1874.
- Steele, D.; Volodin, B.; Savina, O.; Kippelen, B.; Peyghambarian, N.; Röckel, H.; Marder, S. Transillumination imaging through scattering media by use of photorefractive polymers. Opt. Lett. 1998, 23, 153–155.
- Blanche, P.A.; Bablumian, A.; Voorakaranam, R.; Christenson, C.; Lin, W.; Gu, T.; Flores, D.; Wang, P.; Hsieh, W.Y.; Kathaperumal, M.; et al. Holographic three-dimensional telepresence using large-area photorefractive polymer. Nature 2010, 468, 80–83.
- Günter, P.; Huignard, J.P. Photorefractive Materials and Their Applications; Springer: Berlin/Heidelberg, Germany, 2007; Volume 114.
- Blanche, P.A. Photorefractive Organic Materials and Applications; Springer: Berlin/Heidelberg, Germany, 2016; Volume 240.
- Fuentes-Hernandez, C.; Suh, D.J.; Kippelen, B.; Marder, S.R. High-performance photorefractive polymers sensitized by cadmium selenide nanoparticles. Appl. Phys. Lett. 2004, 85, 534–536.
- Tay, S.; Thomas, J.; Eralp, M.; Li, G.; Norwood, R.A.; Schülzgen, A.; Yamamoto, M.; Barlow, S.; Walker, G.A.; Marder, S.R. High-performance photorefractive polymer operating at 1550 nm with near-video-rate response time. Appl. Phys. Lett. 2005, 87, 171105.
- Marder, S.R.; Kippelen, B.; Jen, A.K.Y.; Peyghambarian, N. Design and synthesis of chromophores and polymers for electro-optic and photorefractive applications. Nature 1997, 388, 845–851.
- Thomas, J.; Eralp, M.; Tay, S.; Li, G.; Yamamoto, M.; Norwood, R.A.; Marder, S.R.; Peyghambarian, N.N. Photorefractive polymers with superior performance. Opt. Photonics News 2005, 16, 31.
- Fuentes-Hernandez, C.; Thomas, J.; Termine, R.; Meredith, G.; Peyghambarian, N.; Kippelen, B.; Barlow, S.; Walker, G.; Marder, S.R.; Yamamoto, M.; et al. Video-rate compatible photorefractive polymers with stable dynamic properties under continuous operation. Appl. Phys. Lett. 2004, 85, 1877–1879.
- Eralp, M.; Thomas, J.; Tay, S.; Li, G.; Schülzgen, A.; Norwood, R.; Yamamoto, M.; Peyghambarian, N. Submillisecond response of a photorefractive polymer under single nanosecond pulse exposure. Appl. Phys. Lett. 2006, 89, 114105.
- Kippelen, B.; Meyers, F.; Peyghambarian, N.; Marder, S.R. Chromophore design for photorefractive applications. J. Am. Chem. Soc. 1997, 119, 4559–4560.
- Kippelen, B.; Marder, S.; Hendrickx, E.; Maldonado, J.; Guillemet, G.; Volodin, B.; Steele, D.; Enami, Y.; Yao, Y.; Wang, J.; et al. Infrared photorefractive polymers and their applications for imaging. Science 1998, 279, 54–57.
- Eralp, M.; Thomas, J.; Tay, S.; Li, G.; Meredith, G.; Schülzgen, A.; Peyghambarian, N.; Walker, G.A.; Barlow, S.; Marder, S.R. High-performance photorefractive polymer operating at 975 nm. Appl. Phys. Lett. 2004, 85, 1095–1097.
- Thomas, J.; Fuentes-Hernandez, C.; Yamamoto, M.; Cammack, K.; Matsumoto, K.; Walker, G.A.; Barlow, S.; Kippelen, B.; Meredith, G.; Marder, S.R.; et al. Bistriarylamine Polymer-Based Composites for Photorefractive Applications. Adv. Mater. 2004, 16, 2032–2036.
- Kippelen, B.; Blanche, P.A.; Schülzgen, A.; Fuentes-Hernandez, C.; Ramos-Ortiz, G.; Wang, J.F.; Peyghambarian, N.; Marder, S.R.; Leclercq, A.; Beljonne, D.; et al. Photorefractive Polymers with Non-Destructive Readout. Adv. Funct. Mater. 2002, 12, 615–620.
- Kippelen, B.; Golemme, A.; Hendrickx, E.; Wang, J.F.; Marder, S.R.; Peyghambarian, N. Photorefractive Polymers and Polymer-Dispersed Liquid Crystals. In Field Responsive Polymers; ACS: Washington, DC, USA, 1999; Chapter 14; pp. 204–225.
- Herlocker, J.; Fuentes-Hernandez, C.; Wang, J.; Peyghambarian, N.; Kippelen, B.; Zhang, Q.; Marder, S. Photorefractive polymer composites fabricated by injection molding. Appl. Phys. Lett. 2002, 80, 1156–1158.
- Hendrickx, E.; Herlocker, J.; Maldonado, J.; Marder, S.; Kippelen, B.; Persoons, A.; Peyghambarian, N. Thermally stable high-gain photorefractive polymer composites based on a tri-functional chromophore. Appl. Phys. Lett. 1998, 72, 1679–1681.
- Herlocker, J.; Fuentes-Hernandez, C.; Ferrio, K.; Hendrickx, E.; Blanche, P.A.; Peyghambarian, N.; Kippelen, B.; Zhang, Y.; Wang, J.; Marder, S. Stabilization of the response time in photorefractive polymers. Appl. Phys. Lett. 2000, 77, 2292–2294.
- Hendrickx, E.; Kippelen, B.; Thayumanavan, S.; Marder, S.R.; Persoons, A.; Peyghambarian, N. High photogeneration efficiency of charge-transfer complexes formed between low ionization potential arylamines and C60. J. Chem. Phys. 2000, 112, 9557–9561.
- Masumura, K.; Nakanishi, I.; Khuat, K.V.T.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Optimal composition of the poly (triarylamine)-based polymer composite to maximize photorefractive performance. Sci. Rep. 2019, 9, 1–9.
- Giang, H.N.; Sassa, T.; Fujihara, T.; Tsujimura, S.; Kinashi, K.; Sakai, W.; Wada, S.; Tsutsumi, N. Triphenylamine-Based Plasticizer in Controlling Traps and Photorefractivity Enhancement. ACS Appl. Electron. Mater. 2021, 3, 2170–2177.
- Zhou, P.; Li, Y.; Liu, S.; Su, Y. Colour 3D holographic display based on a quantum-dot-doped liquid crystal. Liq. Cryst. 2019, 46, 1478–1484.
- Wang, Z.; Lv, G.; Feng, Q.; Wang, A.; Ming, H. Resolution priority holographic stereogram based on integral imaging with enhanced depth range. Opt. Express 2019, 27, 2689–2702.
- Fachada, S.; Bonatto, D.; Lafruit, G. High-quality holographic stereogram generation using four RGBD images. Appl. Opt. 2021, 60, A250–A259.
- Liu, J.P.; Lu, S.L. Fast calculation of high-definition depth-added computer-generated holographic stereogram by spectrum-domain look-up table. Appl. Opt. 2021, 60, A104–A110.
