Sarcopenia is a progressive, systemic musculoskeletal disorder associated with an increased risk of adverse events such as falls and fractures, mobility disorders, cardiac and respiratory disease, cognitive impairment, institutionalization, and death. Physical disability and impaired ability to perform activities of daily living contribute to reducing both patient quality of life and functional independence, adding to the necessity of long-term care services for the patient. Considering this evidence, it would seem clear that early diagnosis of sarcopenia and care optimization would also reduce the economic impact on the health care system and individual social-economic burdens.
1. Introduction
Numerous studies and research groups have been working over the past ten years to provide a precise definition of sarcopenia. Five major research groups around the world have developed different definitions and diagnostic criteria: the European Working Group on Sarcopenia in Older People (EWGSOP), the Asia Working Group for Sarcopenia (AWGS), the International Working Group on Sarcopenia (IWGS), the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project, and the Society for Sarcopenia Cachexia and Wasting Disorders (SCWD). As a result, in 2016, sarcopenia was added to the International Classification of Diseases with its ICD-10-MC diagnostic code [
5,
6]. In 2018, the European Working Group on Sarcopenia in Older People (EWGSOP2) published the revised “European Consensus on Sarcopenia” introducing a new working definition, based on low muscle strength as the primary parameter of sarcopenia, considering it a more reliable measure of muscle function and a better predictor of adverse outcomes. Behind sarcopenia, there are mechanisms that in some cases overlap with some cell aging processes. In particular, there are alterations in protein synthesis, proteolysis, neuromuscular integrity, and muscle fat content [
7,
8,
9]. It would be considered that sarcopenia is common in significative fragile patients, often affected by multimorbidity, hospitalization, and consumption of several drugs [
10,
11].
Screening and diagnosis of sarcopenia are important to prevent adverse health outcomes. CT and MRI are the gold standards for noninvasively assessing muscle quantity/mass and identifying adipose tissue [
12,
13]. However, these examinations are expensive, expose patients to radiation, are not always available, and in some situations are difficult to perform and thus must be performed in the hospital. DXA is more easily accessible but less accurate [
1].
In the last few years, new evidence has shown that ultrasound provides detailed imaging about morphology and size and of the surrounding structures [
14]. Several studies reported ultrasound as an appropriate tool to evaluate muscle characteristics and morphology in sarcopenia [
15,
16,
17].
Moreover, ultrasonography is low cost, simple to use, community or hospital-based, is easily repeatable, and is widely used.
The age-related biochemical, anatomical, and physiological changes in muscle tissue have been known for some time. These alterations occur at the molecular and cellular levels and are reflected in macroscopic changes evident on ultrasound. Alterations in muscle architecture reflect underlying sarcopenia, which causes muscle dysfunction. These changes have been recognized as good imaging biomarkers based on ultrasonography [
18].
The quality and quantity of muscle tissue can be easily assessed with ultrasound by using some specific parameters.
In particular, the evaluation of pennate muscles is a potentially valuable tool. In clinical practice, it can highlight even minimal changes over a short period [
19].
Ultrasonography is an accurate method even for use in the elderly, is easy to access, and can also be performed bedside inside the hospital or in the community [
20].
Given the emerging role of ultrasonography, standardization of the method has recently been proposed. However, a global evidence-based consensus is still absent [
21].
Ultrasonography, in the setting of screening and diagnosis of sarcopenia, has the potential to become an imaging-based tool comparable to CT, MRI to quantify body composition on the tissue level, and DXA on the chemical level.
At present, there are no pharmacological treatments available that are effective in the management of sarcopenia [
22], whether they are hormone therapy or drugs. The frontier of sarcopenia treatment, especially in the elderly patient, is a personalized patient-centric approach. It is now known that there is interindividual variability at the cellular level and molecular expression [
23,
24,
25]. This variability underlies alterations in different pathways and whose identity is the basis of the future sarcopenia management. Currently, only an adequate exercise regimen associated with correct nutritional intake (protein) has demonstrated efficacy in the management of sarcopenia. Of utmost importance is the prescription of resistance-based exercise, a diet rich in protein, and with an adequate caloric intake and vitamin supplements [
26].
2. Skeletal Muscle Ultrasound in Sarcopenia
To diagnose sarcopenia in the elderly, evaluating skeletal muscle mass loss is one of the main elements. Although in recent years the importance of associating functional parameters that were to evaluate the loss of muscle performance in the elderly has been emphasized [
1], the diagnosis of sarcopenia cannot ignore the precise measurement of skeletal muscle mass loss, both in quantitative and qualitative terms [
13,
43]. Therefore, the simultaneous evaluation of both these parameters is recommended in the muscle mass measurement phase. On the one hand, diagnostic methods, such as DXA and BIA, are limited to providing only quantitative information about the skeletal muscle structure; on the other hand, CT and MRI, while offering an overview, are burdened by the limitations in the field of everyday clinical practice. In this sense, skeletal muscle ultrasound offers a valid alternative, as it is closely correlated with MRI [
44,
45], with CT [
46] and DXA [
47,
48] in terms of measuring skeletal muscle mass and at the same time offering both quantitative and qualitative information. Although further studies on the standardization of ultrasound measurement methods are needed, the first step in this direction has been provided by the SARCUS study (SARCopenia through UltraSound) [
21].
Ultrasonography (US) can effectively assess the quantity and quality of muscle tissue and has a certain degree of correlation with DXA in muscle study [
48,
49]. Some US parameters measure muscle quantity, such as muscle volume (MV), cross-sectional area (CSA), and muscle thickness (MT). Other parameters describe the muscle qualitatively, for example, echo intensity (EI), pennation angle (PA), fascicle length (FL), and physiologic cross-sectional area (PCSA). There are some experimental approaches, such as the measurement of vascularity.
These parameters can be studied at various “regional sites”; however, the one most frequently used site is the anterior compartment of the thigh. In addition, sarcopenia is not uncommonly site-specific. Muscle loss in the lower limbs is more pronounced in the mentioned district [
50,
51].
The technique involves supine positioning of the subject with legs extended. A linear probe (5–12 MHz) is used, with a fixed gain, and transverse and longitudinal images are taken at the quadriceps femoris. CSA, MT, and EI are assessed on transverse images. PA and FL are measured longitudinally. MT can be performed on any muscle; firstly, the evaluation involves the quadriceps femoris; CSA is evaluated mainly in the rectus femoris. MT and CSA have a good degree of correlation on muscle quantity with the other main methods for studying muscle (MRI, CT, and DXA) [
41,
45,
52]. However, CSA or MT are subject to variability in the muscle explored, to “regional” sarcopenia. Not all anatomical regions undergo muscle loss at the same rate, so there may be some degree of discordance.
Among qualitative measures, muscle echo intensity (EI) is a parameter that has been recently studied. Granted that the amount of muscle does not directly correlate with function [
29,
51], muscle echogenicity provides helpful information about the presence of inflammation, fibrosis, and adipose tissue infiltration [
53]. Increased intramuscular adipose tissue, also referred to as “myostatosis”, is a finding present in both aging-related sarcopenia and cancer cachexia [
54]. These tissue rearrangements can cause an increase in muscle echogenicity, similar to findings in myopathies [
55].
In addition, there appears to be a correlation between echo intensity and muscle strength, gate speed, and sit-to-stand test [
56,
57,
58].
Alterations in architecture are crucial in the genesis of force and are parameters related to muscle function.
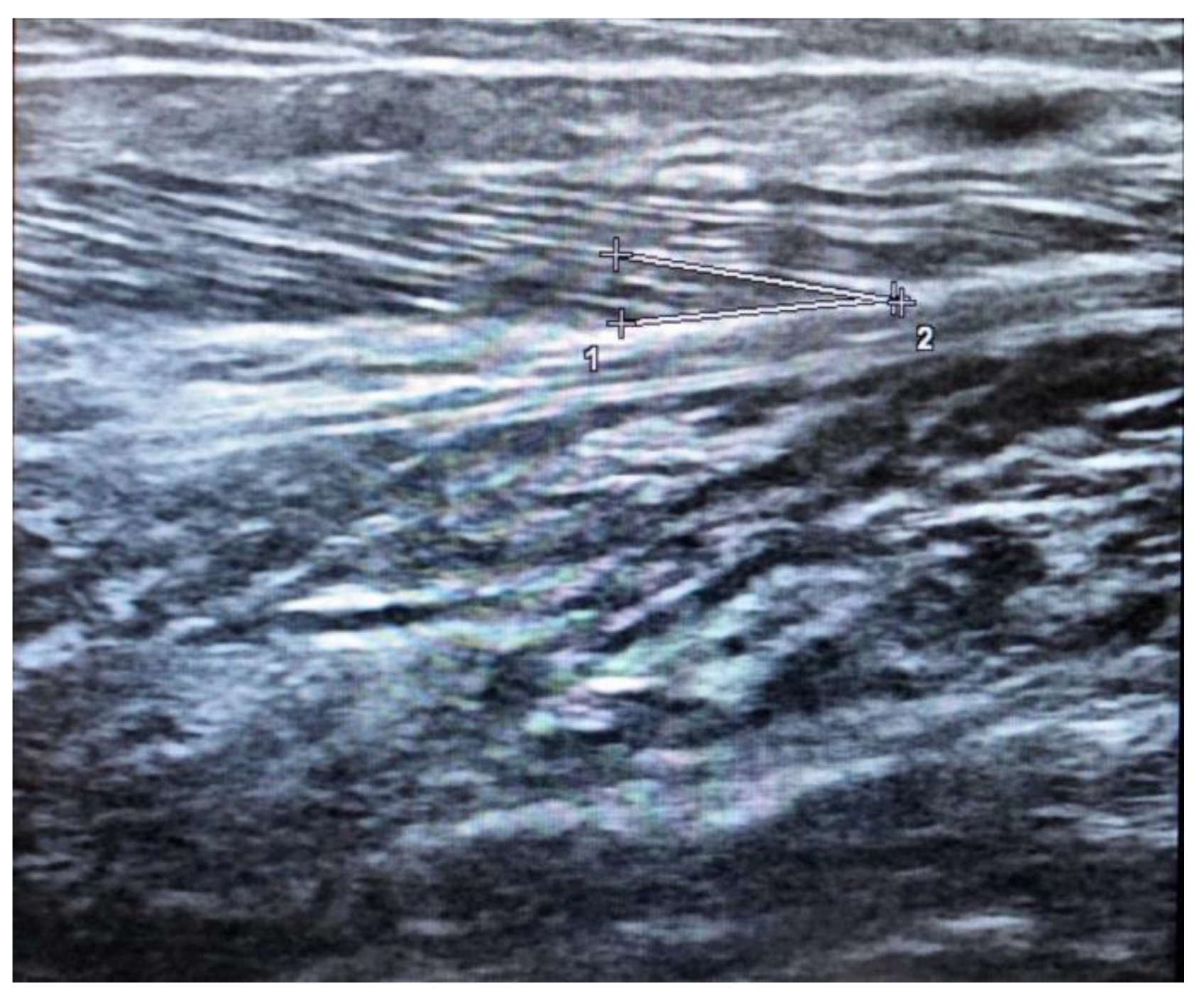
The pennation angle is formed by the insertion of muscle fibers at the level of the deep and superficial aponeurosis in most locomotor muscles, such as the medial gastrocnemius (
Figure 2). The pennation angle and fascicle length are generally evaluated at the level of the gastrocnemius medialis. These two techniques allow for information on the structure and architecture of the muscle and therefore allow for a correlation with the genesis of muscle strength [
30].
3. Conclusions
The application of ultrasound in the assessment of sarcopenia is helpful, especially for detecting muscle failure in older patients not being influenced by the presence of acute and chronic disease and fluid balance. Despite small intra and inter-individual variability, this technique could characterize sarcopenia through the study of qualitative and quantitative parameters measured in different muscles groups, particularly in lower limb muscles. Ultrasound provides a quantitative and qualitative study of muscle, has several modalities, and can study the muscle tissue of multiple anatomical regions. For the evaluation of sarcopenia, state of the art ultrasonography had the potential to be comparable to the other main methodologies. The recent attempts to standardize the technique, the low cost, the simplicity of execution, the absence of ionizing radiation, the reproducibility, and repeatability make it a possible method to be used in the near future for the diagnostic assessment and treatment response in sarcopenia. Further studies will be needed to confirm and standardize the use of the methodology in a clinical setting.
In conclusion, having limited options for sarcopenia management, the best strategy is prevention and early diagnosis. With this perspective, determining the future role of ultrasound in this field will be crucial.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10235552