Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Mycology
The rise of the holobiont concept confers a prominent importance to the endophytic associates of plants, particularly to species known to be able to exert a mutualistic role as defensive or growth-promoting agents. The finding that many entomopathogenic fungi are harbored within plant tissues and possess bioactive properties going beyond a merely anti-insectan effect has recently prompted a widespread investigational activity concerning their occurrence and functions in crops, in the aim of an applicative exploitation conforming to the paradigm of sustainable agriculture.
- entomopathogens
- endophytic fungi
- crop protection
- plant growth promotion
- integrated pest management
- Cordycipitaceae
- plant mycobiome
1. Introduction
The great microbial diversity harbored in plants has just started being explored in light of a consolidated awareness that what we manage in the agricultural practice is actually the outcome of the combined expression of plant and microbial genes [1][2]. The symbiotic relationships between endophytic fungi and their host plants exteriorize in many ways, ranging from opportunistic saprophytism in senescent tissues, to latent pathogenicity disclosing after the impact of various stress factors, to genuine mutualistic interactions deriving from nutritional support and/or increased protection against pests and pathogens. The latter are particularly relevant for the holistic approach making its way in integrated pest management (IPM), considering the crop production system as a whole in the aim to contain rather than eradicate pests.
Within this conceptual rearrangement, the improvement of our knowledge on occurrence and functions of endophytic associates of plants is fundamental in view of their possible exploitation in sustainable agriculture. Endophytic entomopathogens are an important category of the plant microbiome, which is increasingly considered for applicative purposes. So far, the majority of investigations and reports concerning these organisms deal with Beauveria bassiana and Metarhizium anisopliae, with several fine reviews available in the literature [3][4].
2. Taxonomic Background
Until the early 2000s, these fungi were classified in the section Prostrata of the genus Verticillium, basically with reference to their imperfect stage producing verticillate conidiophores [5]. A few species best known for their parasitic behavior against arthropods, nematodes and/or fungi were ascribed to this section, such as V. chlamydosporium, V. lecanii and V. psalliotae. Afterwards, the application of biomolecular techniques enabled to shed light on the phylogenetic relationships within this heterogeneous genus. Particularly, species within the section Prostrata were separated in a few unrelated genera, such as Pochonia, Haptocillium, Simplicillium and Lecanicillium, and their teleomorphs identified within the genera Cordyceps and Torrubiella [6]. The species V. fungicola, previously ascribed to the section Albo-erecta in the genus Verticillium, was later aggregated to Lecanicillium [7]. As a result of this fundamental revision, about fifteen Lecanicillium species were recognized, a few of which (L. attenuatum, L. longisporum, L. muscarium, L. nodulosum and L. lecanii s.str.) enucleated from the previously collective V. lecanii.
However, as it often happens in fungal taxonomy, such a sound rearrangement was not destined to persist. In fact the genus Lecanicillium was shown to be paraphyletic [8], and some species were moved to Akanthomyces, a pre-existing but overlooked genus including entomogenous species [9] (Table 1). At the same time, investigations in more or less peculiar ecological contexts brought to the description of novel taxa of both Akanthomyces and Lecanicillium [10][11], while some species ascribed to the latter genus, such as L. uredinophilum and L. pissodis, were shown to actually fit in the A. lecanii clade [12]. Following the dismissal of the dual nomenclature system for pleomorphic fungi, a more comprehensive revision of the whole family of the Cordycipitaceae is in progress. Particularly, rejection has been proposed for the genus name Lecanicillium, while some Akanthomyces species have in turn been moved to another genus (Hevansia) [13]. Hence, further adjustments concerning species still classified in Lecanicillium are to be expected.
Table 1. Nomenclatural correspondence of accepted Lecanicillium/Akanthomyces species with sequences of internal transcribed spacers of ribosomal DNA (rDNA-ITS) available in GenBank.
| Species Names * | ITS Sequence Used in | ||
|---|---|---|---|
| Lecanicillium | Akanthomyces | Cordyceps/Torrubiella | Phylogenetic Analysis |
| L. acerosum | NR11268 | ||
| L. antillanum | AJ292392 | ||
| L. aphanocladii | LT220701 | ||
| L. aranearum | A. aranearum | T. alba | AJ292464 |
| L. araneicola | AB378506 | ||
| L. araneogenum | A. neoaraneogenus | NR161115 | |
| L. attenuatum | A. attenuatus | AJ292434 | |
| L. cauligalbarum | MH730663 | ||
| L. coprophilum | MH177615 | ||
| L. dimorphum | AJ292429 | ||
| L. flavidum | EF641877 | ||
| L. fungicola var.aleophilum | NR111064 | ||
| L. fungicola var.fungicola | NR119653 | ||
| L. fusisporum | AJ292428 | ||
| L. kalimantanense | AB360356 | ||
| L. lecanii | A. lecanii | C. confragosa | AJ292383 |
| L. longisporum | A. dipterigenus | AJ292385 | |
| L. muscarium | A. muscarius | NR111096 | |
| L. nodulosum | Akanthomyces sp. | EF513012 | |
| L. primulinum | NR119418 | ||
| L. psalliotae | AJ292389 | ||
| L. restrictum | LT548279 | ||
| L. sabanense | A. sabanensis | KC633232 | |
| L. subprimulinum | MG585314 | ||
| L. tenuipes | AJ292391 | ||
| L. testudineum | LT548278 | ||
| L. uredinophilum | Akanthomyces sp. | MG948305 | |
| L. wallacei | T. wallacei | NR111267 | |
| Lecanicillium sp. | C. militaris | AF153264 | |
| A. aculeatus | KC519371 | ||
| A. coccidioperitheciatus | C. coccidioperitheciata | JN049865 | |
| A. kanyawimiae | MF140751 | ||
| A. sphingum | C. sphingum | AY245641 | |
| A. sulphureus | Torrubiella sp. | MF140756 | |
| A. thailandicus | Torrubiella sp. | MF140755 | |
| A. tuberculatus | C. tuberculata | JN049830 | |
| A. waltergamsii | MF140747 | ||
* The currently used species names as inferred from the Mycobank database [14] are reported in bold.
3. Occurrence
The number of reports concerning endophytic isolates of Lecanicillium and Akanthomyces has increased in recent years. This is due not only to the several taxonomic reassessments introducing new species, but also to the easier access to techniques and databases for DNA sequencing, which in most instances enable one to overcome the intrinsic difficulties of morphological identification. However, more prompts have probably resulted by the awareness of the basic role that endophytic fungi play on plant fitness, introducing applicative perspectives for investigations in the field. For the above genera, literature shows a prevalence of findings concerning natural phytocoenoses (Table 2) over those inherent crops (Table 3); even more so considering that the latter series includes a few cases of endophytic colonization resulting after artificial inoculation in experimental work. Basically connected with the issue of ecosystem simplification characterizing the agricultural contexts, such a difference emphasizes the opportunity to recover the functional role of this component of the plant holobiont in view of improving crop performances.
Table 2. Endophytic occurrence of Lecanicillium/Akanthomyces in wild contexts.
| Species | Host Plant | Country | ITS Sequence √ | Reference |
|---|---|---|---|---|
| A. attenuatus | Astrocaryum sciophilum | French Guyana | MK279520 | [15] |
| Conifer plant | China | MN908945 | GenBank | |
| Symplocarpus foetidus | Canada | KC916681 | [16] | |
| A. lecanii | Ammophila arenaria | Spain | - | [17] |
| Dactylis glomerata | Spain | AM262369 | [18] | |
| Deschampsia flexuosa | Finland | KJ529005 | [19] | |
| Elymus farctus | Spain | AM924163 | [17] | |
| Laretia acaulis | Chile | - | [20] | |
| Pinus sylvestris | Italy | KJ093501 | [21] | |
| Pinus sylvestris | Poland | - | [22] | |
| Shorea thumbuggaia | India | KJ542654 | GenBank | |
| Taxus baccata | Iran | KF573987 | [23] | |
| A. muscarius | Acer campestre | Italy | MT230457 | This paper |
| Laurus nobilis | Italy | - | [24] | |
| Myrtus communis | Italy | MT230435 | This paper | |
| Nypa fruticans | Thailand | MH497223 | [25] | |
| Quercus robur | Italy | MT230463 | This paper | |
| Akanthomyces sp. * | Arctostaphylos uva-ursi | Switzerland | - | [26] |
| Carpinus caroliniana | USA | - | [27] | |
| L. aphanocladii | Ageratina adenophora | China | MK304090 MK304173 MK304418 |
[28] |
| Hemidesmus indicus | India | MH594215 | [29] | |
| Huperzia serrata | China | KP689216 KP689173 |
[30] | |
| Picea mariana | Canada | - | [31] | |
| L. fungicola | Phragmites australis | Korea | KP017880 | [32] |
| L. kalimantanense | Zingiber officinale | Indonesia | - | [33] |
| L. psalliotae | Cerastium fischerianum | Korea | JX238776 | [34] |
| Coix lachryma-jobi | China | KJ572167 | GenBank | |
| Magnolia officinalis | China | GenBank | ||
| Phoradendron perrottettii | Brazil | - | [35] | |
| Pinus radiata | New Zealand | - | [36] | |
| Sedum oryzifolium | Korea | KU556134 | [37] | |
| Tapirira guianensis | Brazil | - | [35] | |
| Triticum dicoccoides | Israel | - | [38] | |
| Lecanicillium sp. | Artocarpus lacucha | India | MH700423 MH700428 |
GenBank |
| Bupleurum chinense | China | MG561939 | GenBank | |
| Huperzia serrata | China | KM513600 | [30] | |
| Liparis japonica | China | KT719186 KT719187 KT719188 KT719189 KT719192 |
GenBank | |
| Micrandra spruceana | Peru | MH267985 | [39] | |
| Microthlaspi perfoliatum | Greece | KT269776 | [40] | |
| Quassia indica | India | MH910098 | GenBank | |
| Sandwithia guyanensis | French Guyana | MN514023 | [41] | |
| Theobroma gileri | Ecuador | - | [42] |
√ Missing ITS accession number implies identification based on morphological characters only, or without depositing the ITS sequence. * These strains were originally identified as Verticillium lecanii.
Table 3. Endophytic occurrence of Lecanicillium/Akanthomyces in crops.
| Species | Host Plant | Country | ITS Sequence √ | Reference |
|---|---|---|---|---|
| A. attenuatus | Brachiaria sp. | Kenya | KU574698 | [43] |
| Salvia miltiorrhiza | China | JX406555 | GenBank | |
| A. lecanii | Cucurbita maxima | Australia | - | [44] |
| Gossypium hirsutum | Australia | - | [45] | |
| Gossypium hirsutum | Brazil | - | [46] | |
| Gossypium hirsutum | Texas, USA | KP407570 | [47] | |
| Solanum lycopersicum | Australia | - | [44] | |
| Phaseolus vulgaris | Australia | - | [44] | |
| Phaseolus vulgaris | China | - | [48] | |
| Pistacia vera | Iran | MF000354 | [49] | |
| Triticum aestivum | Australia | - | [44] | |
| Vitis vinifera | Spain | - | [50] | |
| Zea mays | Australia | - | [44] | |
| A. muscarius | Brassica oleracea | New Zealand | - | [51] |
| Cucumis sativus | Canada | - | [52] | |
| Cucumis sativus | Japan | - | [53] | |
| Prunus cerasus | Iran | KY472303 | [54] | |
| L. aphanocladii | Zea mays | Slovenia | - | [55] |
| L. dimorphum | Phoenix dactylifera | Spain | - | [56] |
| L. psalliotae | Phoenix dactylifera | Spain | - | [56] |
| Lecanicillium sp. | Citrus limon | Iran | MN448344 | GenBank |
| Vitis vinifera | China | MT123107 | GenBank | |
| Zea mays | India | - | [57] |
√ Missing ITS accession number implies identification based on morphological characters only, or without depositing the ITS sequence.
Overall, Table 2 and Table 3 include 65 citations of endophytic strains belonging to these two genera as a result of a search considering literature in the field and the GenBank database. A widespread capacity to colonize plants from heterogeneous ecological contexts is evident considering that these citations refer to 54 species belonging to 35 botanical families. With 10 species Poaceae is the most represented family, followed by Arecaceae and Pinaceae with three species each, and Anacardiaceae, Apiaceae, Brassicaceae, Cucurbitaceae, Euphorbiaceae and Malvaceae with two species. The rest of the families (Apocynaceae, Araceae, Asteraceae, Betulaceae, Caryophyllaceae, Crassulaceae, Dipterocarpaceae, Ericaceae, Fabaceae, Fagaceae, Lamiaceae, Lauraceae, Lycopodiaceae, Magnoliaceae, Moraceae, Myrtaceae, Orchidaceae, Rosaceae, Rutaceae, Santalaceae, Sapindaceae, Simaroubaceae, Solanaceae, Taxaceae, Vitaceae and Zingiberaceae) are represented by a single species.
Such a variety of hosts seems to contrast any hypothesis of host specialization, and is rather indicative of a possible tendency to spread horizontally within the phytocoenoses. In this respect, the recovery of A. muscarius from four woody species (Acer campestre, Laurus nobilis, Quercus robur and Myrtus communis in two separate stands) at the Astroni Nature Reserve near Napoli, Italy ([24] and in this paper), appears to support this ability, which may as well imply a permanent functional role in natural ecosystems. On the other hand, indications of a constant association with crop species could be favorable for possible applications in IPM. The limited available data only support preliminary clues in the case of cotton (Gossypium hirsutum) where, considering the economic impact of insect pests, the endophytic occurrence of strains of A. lecanii reported from distant countries such as Australia, Brazil and the United States might deserve further attention.
Phylogenetic Relationships of Endophytic Strains
In the evolving taxonomic scheme outlined above, the endophytic isolates provisionally classified as Lecanicillium sp. are to be further considered for a more definite taxonomic assignment. In this perspective, we propose a phylogenetic analysis (Figure 1) considering strains whose sequences of internal transcribed spacers of ribosomal DNA (rDNA-ITS) are deposited in GenBank (Table 2 and Table 3), along with official reference strains for the currently accepted species of Lecanicillium and Akanthomyces (Table 1).
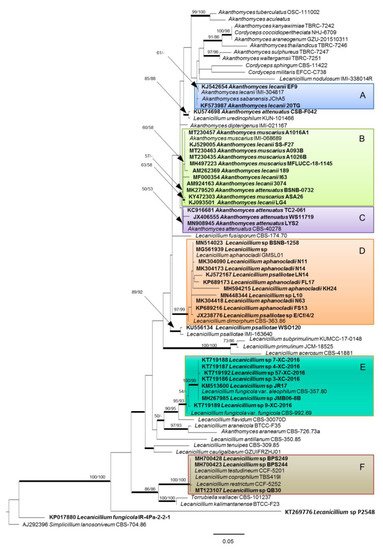
Figure 1. Phylogenetic tree based on maximum likelihood (ML) analysis of the rDNA-ITS sequences deposited in GenBank for the known species (Table 1) and the endophytic strains of Lecanicillium and Akanthomyces (in bold, Table 2 and Table 3). Multiple sequence alignment comprised 592 nucleotide positions, including gaps. The analysis was carried out using RAxML software (version 8.2.12; https://cme.h-its.org/exelixis/web/software/raxml) for ML, PAUP (version 4.0a166; https://paup.phylosolutions.com) for maximum parsimony (MP), and MrBayes (version 3.2.7a; https://nbisweden.github.io/MrBayes/download.html) for Bayesian analysis. Phylogenetic tree was drawn using FigTree software (version 1.4.4; http://tree.bio.ed.ac.uk/software/figtree). Details and complete references are specified in a recent paper [58]. Bootstrap support values ≥60% for ML and MP are presented above branches as follows: ML/MP, bootstrap support values <50% are marked with ‘-’. Branches in bold are supported by Bayesian analysis (posterior probability ≥95%). Simplicillium lanosoniveum CBS 704.86 (GenBank: AJ292396) was used as outgroup reference. Main clades are indicated by colored boxes A, B, C, D, E and F.
Although more DNA sequences, such as the translation elongation factor 1 alpha (TEF) and RNA polymerase II largest subunits 1 (RPB1) and 2 (RPB2), are considered in taxonomic assessments concerning genera in the Cordycipitaceae [12][13][25][59], provisional identification of isolates recovered in the course of biodiversity studies is routinely done on account of ITS. Therefore only these kinds of sequences are usually deposited in GenBank for such strains, representing the only possible marker available for phylogenetic reconstructions.
In the absence of opportunities for a direct examination of these isolates, the phylogenetic tree proposed in Figure 1 provides an indication for their provisional assimilation to any of the accepted taxa in the genera Lecanicillium and Akanthomyces. A major cluster in the upper part of the tree includes the type strains of the species of Cordyceps, Akanthomyces (except A. aranearum), and of L. nodulosum and L. uredinophilum, which are also credited for ascription to Akanthomyces, along with all the endophytic strains ascribed to the species A. lecanii, A. muscarius and A. attenuatus (clades A, B and C, respectively). However, just two out of seven endophytic isolates ascribed to A. lecanii are next to the type strain of this species, while five more isolates rather group with A. muscarius. Confirming evidence from previous phylogenetic analyses [25][59], A. attenuatus is very close to A. muscarius, but an isolate from the palm Astrocaryum sciophilum is displaced in clade B. Another isolate from Brachiaria sp. reported as A. attenuatus is more distant, having L. uredinophilum as the closest relative. While these remarks cannot be taken as an evidence of a more common endophytic occurrence of A. muscarius, they represent an indication that at least some isolates of this species might have been misidentified as A. lecanii. This is not surprising, considering that a previous study pointed out the difficulty of resolving species ascription of strains previously ascribed to V. lecanii by using ITS sequences only [60].
Interestingly, no endophytic isolates provisionally identified as Lecanicillium sp. belong to the above major Akanthomyces cluster. Three of them are part of clade D, corresponding to the species L. aphanocladii, which also includes two strains identified as L. psalliotae. This is acceptable since these species and L. dimorphum have been reported in a close phylogenetic relationship in previous analyses [6][59]. However, L. psalliotae seems somehow problematic with reference to the resolution power of ITS, considering that it was reported as the closest relative (99.65% sequence identity at 100% query cover) of another isolate from Microthlaspi perfoliatum [40], which is in a quite distant position in our phylogenetic tree.
As many as seven unidentified strains cluster with L. fungicola, prevalently with the type strain of var. aleophilum (clade E), indicating a relevant endophytic occurrence of this species, which was not recognized so far. Another isolate reported as L. fungicola [32], deserves a more careful consideration with reference to its basal placement. In fact, BLAST search in GenBank indicated a 100% identity with ten strains of this species and several strains of the unrelated Simplicillium aogashimaense. The latter was characterized in 2013 with the support of a phylogenetic analysis based on ITS only, which anyway showed a consistent distance from L. fungicola [61]. Quite meaningfully, in our analysis the isolate in question was placed in proximity to the outgroup (Simplicillium lanosoniveum) on which our tree was rooted. Considering that sequences of six out of this group of ten L. fungicola strains were deposited in GenBank before 2013, it is quite possible that original misidentification of those that might rather have been Simplicillium strains could have determined the incorrect assignment of the more recent isolates.
Finally, three isolates (two Indian from Artocarpus lacucha and one Chinese from Vitis vinifera) are grouped in clade F together with the type strains of the recently described L. coprophilum [11], L. restrictum and L. testudineum [62]. A BLAST search in the GenBank database shows the first species as the closest relative, with 100% and 99.81% ITS sequence identity for the Chinese and the Indian isolates, respectively.
4. Implications in Crop Protection
As introduced above, so far there are few observations concerning the effects of endophytic strains of Lecanicillium and Akanthomyces in crops. Within the limited data available so far, cotton stands out for remarks on the endophytic occurrence of A. lecanii from independent cropping areas. In Australia an endophytic isolate was shown to be able to colonize cotton plants ensuring protection against the cotton aphid (Aphis gossypii) after artificial inoculation. Besides evidence from direct microscopic examination, the ability to colonize plant tissues was confirmed by re-isolation from leaves of the treated plants, which was successful up to 35 days after inoculation. This persistence can be taken as an indication of an endophytic life strategy, considering that endophytic colonization enables the fungus to become resident in a stable and nutritious insect-attracting environment. High humidity enhanced colonization of both plants and aphids; this expected effect is relevant for the management of the cotton aphid, which is most commonly found in the lower canopy, where humidity is high and the fungus is more protected against the adverse effects of UV radiation from sun [63]. Moreover, contact with conidia of A. lecanii significantly reduced the rate and period of reproduction of A. gossypii. The culture filtrate of the fungus significantly increased mortality and reduced reproduction, while feeding-choice experiments indicated that the aphids might be able to detect the fungal metabolites. The ethyl acetate and methanolic fractions of culture filtrate and mycelia also caused significant mortality and reduced fecundity [64]. Besides cotton, the same strain displayed the ability to colonize plants of wheat, corn, tomato, bean and pumpkin after artificial inoculation of leaves, while soil inoculation was ineffective [44].
Additional reports from cotton come from Texas [47] and Brazil, where the endophytic occurrence of A. lecanii was detected in leaves and roots of both normal and Bt-transgenic plants [46]. Although no aspects concerning interactions with pests were evaluated in these cases, it is meaningful that several strains of A. lecanii were recovered in each of these three contexts, indicating a possible common association of this species with cotton, which deserves to be more thoroughly verified.
The adaptation of A. lecanii to exert entomopathogenicity in association with plants is well attested by the finding that the fungus responds to volatile compounds produced by the plant during insect feeding. Particularly, in a model based on thale cress (Arabidopsis thaliana) and the mustard aphid (Lipaphis erysimi), compounds such as methyl salicylate and menthol were found to promote spore germination and pathogenicity of the fungus [65][66].
Besides aphids, protective effects after systemic colonization have been demonstrated against the red spider mite (Tetranychus urticae) in bean plants. In this case a strain of A. lecanii was reported to spread within the plant tissues after artificial inoculation of seeds, promoting growth and impairing survival and fecundity of the mites. These effects were even carried over the following generation of mites fed on fresh plants [48].
Pathogenicity of A. lecanii against a wide array of noxious arthropods is integrated by antagonism towards plant pathogenic fungi. In addition to a general antifungal activity demonstrated in vitro against polyphagous species such as Sclerotinia sclerotiorum, Rhizoctonia solani and Aspergillus flavus [49], possible exploitation of this double functionality has been conceived on several crops, such as coffee where A. lecanii behaves as both a parasite of the leaf rust (Hemileia vastatrix) and a pathogen of the green scale (Coccus viridis) [67]. The same role can be considered in crops where powdery mildews can represent a major phytosanitary problem, such as cucurbits [68][69].
Moreover, antifungal effects could derive from stimulation of the plant defense response, as reported for an endophytic strain able to promote such reaction against Pythium ultimum in transformed cucumber plants [52]. Additional experimental evidence in this regard is provided by observations carried out on the date palm (Phoenix dactylifera) where the inoculation of endophytic strains of L. dimorphum and L. cf. psalliotae, previously reported for entomopathogenicity against the red palm scale (Phoenicococcus marlatti) [56], induced proteins involved in plant defense or stress response. Proteins related with photosynthesis and energy metabolism were also upregulated, along with accumulation of a heavy chain myosin-like protein [70].
The concurrent role against plant pests and pathogens is known to operate for other Lecanicillium and Akanthomyces species, and for non-endophytic strains of various origin, as more in detail discussed in dedicated papers [71][72]. The need to combat multiple adversities has also prompted the evaluation of a possible combined use of these fungi with chemical pesticides. In this respect, it has been observed that the spread of A. lecanii in plant tissues is not affected by treatments with insecticides belonging to several classes [73]. Moreover, substantial safety of insecticides has been reported in in vitro assays carried out on A. muscarius, while several herbicides and fungicides were responsible for negative effects or even suppression of mycelial growth [74]. For the latter species, in vivo observations on the sweet potato whitefly (Bemisia tabaci) demonstrated the positive effects of association with chemical insecticides in view of reducing their use, particularly in the greenhouse [75]. Again with reference to application of A. muscarius for the control of B. tabaci, it is worth mentioning the synergistic effects resulting in combined treatments with matrine, a plant-derived quinolizidine alkaloid [76].
In addition to the indirect side effects deriving from protection against biotic and abiotic adversities, many endophytes have been reported to promote plant growth through essentially two mechanisms; that is the release of plant hormones, or the improvement of nutritional conditions. Of course, strains possessing both properties are likely to contribute in an additive manner, as observed for an isolate of L. psalliotae from cardamom (Elettaria cardamomum). Besides producing indole-3-acetic acid, this strain enhanced chlorophyll content of leaves as a likely result of release of siderophores, and increased availability of zinc and inorganic phosphate by promoting their solubilization [77]. Release of siderophore has also been reported for an endophytic isolate of A. lecanii from Pistacia vera [49].
This entry is adapted from the peer-reviewed paper 10.3390/agriculture10060205
References
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant. Mol. Biol. 2016, 90, 645–655.
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58.
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. BioControl 2017, 62, 1–17.
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30.
- Gams, W.; Van Zaayen, A. Contribution to the taxonomy and pathogenicity of fungicolous Verticillium species. I. Taxonomy. Neth. J. Plant. Pathol. 1982, 88, 57–78.
- Zare, R.; Gams, W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwig. 2001, 73, 1–50.
- Zare, R.; Gams, W. A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol. Res. 2008, 112, 811–824.
- Sung, G.H.; Hywel-Jones, N.J.; Sung, J.M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59.
- Mains, E.B. Entomogenous species of Akanthomyces, Hymenostilbe and Insecticola in North America. Mycologia 1950, 42, 566–589.
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-ard, J. Disentangling cryptic species with Isaria-like morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257.
- Su, L.; Zhu, H.; Guo, Y.; Du, X.; Guo, J.; Zhang, L.; Qin, C. Lecanicillium coprophilum (Cordycipitaceae, Hypocreales), a new species of fungus from the feces of Marmota monax in China. Phytotaxa 2019, 387, 55–62.
- Wei, D.P.; Wanasinghe, D.N.; Chaiwat, T.A.; Hyde, K.D. Lecanicillium uredinophilium known from rusts, also occurs on animal hosts with chitinous bodies. Asian J. Mycol. 2018, 1, 63–73.
- Kepler, R.M.; Luangsa-ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335.
- Mycobank Database. Available online: http://www.mycobank.org/ (accessed on 9 April 2020).
- Barthélemy, M.; Elie, N.; Pellissier, L.; Wolfender, J.L.; Stien, D.; Touboul, D.; Eparvier, V. Structural identification of antibacterial lipids from Amazonian palm tree endophytes through the molecular network approach. Int. J. Mol. Sci. 2019, 20, 2006.
- Ellsworth, K.T.; Clark, T.N.; Gray, C.A.; Johnson, J.A. Isolation and bioassay screening of medicinal plant endophytes from eastern Canada. Can. J. Microbiol. 2013, 59, 761–765.
- Sánchez-Márquez, S.; Bills, G.F.; Zabalgogeazcoa, I. Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses. Fungal Divers. 2008, 33, 87–100.
- Sánchez Márquez, M.; Bills, G.F.; Zabalgogeazcoa, I. The endophytic mycobiota of the grass Dactylis Glomerata. Fungal Divers. 2007, 27, 171–195.
- Poosakkannu, A.; Nissinen, R.; Kytöviita, M.M. Culturable endophytic microbial communities in the circumpolar grass, Deschampsia flexuosa in a sub-Arctic inland primary succession are habitat and growth stage specific. Environ. Microbiol. Rep. 2015, 7, 111–122.
- Molina-Montenegro, M.A.; Oses, R.; Torres-Díaz, C.; Atala, C.; Núñez, M.A.; Armas, C. Fungal endophytes associated with roots of nurse cushion species have positive effects on native and invasive beneficiary plants in an alpine ecosystem. Perspect. Plant. Ecol. Evol. Syst. 2015, 17, 218–226.
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, e83.
- Behnke-Borowczyk, J.; Kwaśna, H.; Kulawinek, B. Fungi associated with Cyclaneusma needle cast in Scots pine in the west of Poland. For. Pathol. 2019, 49, e12487.
- Ashkezari, S.J.; Fotouhifar, K.B. Diversity of endophytic fungi of common yew (Taxus baccata L.) in Iran. Mycol. Progr. 2017, 16, 247–256.
- Nicoletti, R.; De Filippis, A.; Buommino, E. Antagonistic aptitude and antiproliferative properties on tumor cells of fungal endophytes from the Astroni Nature Reserve, Italy. Afr. J. Microbiol. Res. 2013, 7, 4073–4083.
- Vinit, K.; Doilom, M.; Wanasinghe, D.N.; Bhat, D.J.; Brahmanage, R.S.; Jeewon, R.; Xiao, Y.; Hyde, K.D. Phylogenetic placement of Akanthomyces muscarius, a new endophyte record from Nypa fruticans in Thailand. Curr. Res. Environ. Appl. Mycol. 2018, 8, 404–417.
- Widler, B.; Müller, E. Untersuchungen über endophytische pilze von Arctostaphylos uva-ursi (L.) Sprengel (Ericaceae). Bot. Helv. 1984, 94, 307–337.
- Bills, G.F.; Polishook, J.D. Microfungi from Carpinus caroliniana. Can. J. Bot. 1991, 69, 1477–1482.
- Fang, K.; Miao, Y.F.; Chen, L.; Zhou, J.; Yang, Z.P.; Dong, X.F.; Zhang, H.B. Tissue-specific and geographical variation in endophytic fungi of Ageratina adenophora and fungal associations with the environment. Front. Microbiol. 2019, 10, 2919.
- Shobha, M.; Bharathi, T.R.; Sampath Kumara, K.K.; Prakash, H.S. Diversity and biological activities of fungal root endophytes of Hemidesmus indicus (L.) R.Br. J. Pharmacogn. Phytochem. 2019, 8, 273–280.
- Wang, Y.; Lai, Z.; Li, X.X.; Yan, R.M.; Zhang, Z.B.; Yang, H.L.; Zhu, D. Isolation, diversity and acetylcholinesterase inhibitory activity of the culturable endophytic fungi harboured in Huperzia serrata from Jinggang Mountain, China. World J. Microbiol. Biotechnol. 2016, 32, 20.
- Summerbell, R.C. Root endophyte and mycorrhizosphere fungi of black spruce, Picea mariana, in a boreal forest habitat: Influence of site factors on fungal distributions. Stud. Mycol. 2005, 53, 121–145.
- Khalmuratova, I.; Kim, H.; Nam, Y.J.; Oh, Y.; Jeong, M.J.; Choi, H.R.; You, Y.H.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; et al. Diversity and plant growth promoting capacity of endophytic fungi associated with halophytic plants from the west coast of Korea. Mycobiology 2015, 43, 373–383.
- Ginting, R.C.B.; Sukarno, N.; Widyastuti, U.; Darusman, L.K.; Kanaya, S. Diversity of endophytic fungi from red ginger (Zingiber officinale Rosc.) plant and their inhibitory effect to Fusarium oxysporum plant pathogenic fungi. HAYATI J. Biosci. 2013, 20, 127–137.
- Kim, H.; You, Y.H.; Yoon, H.; Seo, Y.; Kim, Y.E.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; Kim, J.G. Culturable fungal endophytes isolated from the roots of coastal plants inhabiting Korean east coast. Mycobiology 2014, 42, 100–108.
- de Abreu, L.M.; Almeida, A.R.; Salgado, M.; Pfenning, L.H. Fungal endophytes associated with the mistletoe Phoradendron perrottettii and its host tree Tapirira guianensis. Mycol. Progr. 2010, 9, 559–566.
- Brownbridge, M.; Reay, S.D.; Nelson, T.L.; Glare, T.R. Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control. 2012, 61, 194–200.
- You, Y.H.; Park, J.M.; Seo, Y.G.; Lee, W.; Kang, M.S.; Kim, J.G. Distribution, characterization, and diversity of the endophytic fungal communities on Korean seacoasts showing contrasting geographic conditions. Mycobiology 2017, 45, 150–159.
- Ofek-Lalzar, M.; Gur, Y.; Ben-Moshe, S.; Sharon, O.; Kosman, E.; Mochli, E.; Sharon, A. Diversity of fungal endophytes in recent and ancient wheat ancestors Triticum dicoccoides and Aegilops sharonensis. FEMS Microbiol. Ecol. 2016, 92, fiw152.
- Skaltsas, D.N.; Badotti, F.; Vaz, A.B.M.; da Silva, F.F.; Gazis, R.; Wurdack, K.; Castlebury, L.; Góes-Neto, A.; Chaverri, P. Exploration of stem endophytic communities revealed developmental stage as one of the drivers of fungal endophytic community assemblages in two Amazonian hardwood genera. Sci. Rep. 2019, 9, 12685.
- Glynou, K.; Ali, T.; Buch, A.K.; Haghi Kia, S.; Ploch, S.; Xia, X.; Çelik, A.; Thines, M.; Maciá-Vicente, J.G. The local environment determines the assembly of root endophytic fungi at a continental scale. Environ. Microbiol. 2016, 18, 2418–2434.
- Mai, P.Y.; Levasseur, M.; Buisson, D.; Touboul, D.; Eparvier, V. Identification of antimicrobial compounds from Sandwithia guyanensis-associated endophyte using molecular network approach. Plants 2020, 9, 47.
- Evans, H.C.; Holmes, K.A.; Thomas, S.E. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol. Progr. 2003, 2, 149–160.
- Kago, L.; Njuguna, J.; Njarui, D.M.G.; Ghimire, S.R. Fungal endophyte communities of Brachiaria grass (Brachiaria spp.) in Kenya. In Climate Smart Brachiaria Grasses for Improving Livestock Production in East Africa—Kenya Experience: Proceedings of the Workshop, Naivasha, Kenya, 14–15 September 2016; Njarui, D.M.G., Gichangi, E.M., Ghimire, S.R., Muinga, R.W., Eds.; Kenya Agricultural and Livestock Research Organization: Nairobi, Kenya, 2016; pp. 150–162.
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control. 2010, 55, 34–41.
- McGee, P.A. Reduced growth and deterrence from feeding of the insect pest Helicoverpa armigera associated with fungal endophytes from cotton. Aus. J. Experim. Agric. 2002, 42, 995–999.
- de Souza Vieira, P.D.; de Souza Motta, C.M.; Lima, D.; Torres, J.B.; Quecine, M.C.; Azevedo, J.L.; de Oliveira, N.T. Endophytic fungi associated with transgenic and non-transgenic cotton. Mycology 2011, 2, 91–97.
- Castillo-Lopez, D. Ecological roles of two entomopathogenic endophytes: Beauveria bassiana and Purpureocillium lilacinum in cultivated cotton. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 11 May 2015.
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L. (Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392.
- Dolatabad, H.K.; Javan-Nikkhah, M.; Shier, W.T. Evaluation of antifungal, phosphate solubilisation, and siderophore and chitinase release activities of endophytic fungi from Pistacia vera. Mycol. Progr. 2017, 16, 777–790.
- González, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42.
- Kuchár, M.; Glare, T.R.; Hampton, J.G.; Dickie, I.A.; Christey, M.C. Virulence of the plant-associated endophytic fungus Lecanicillium muscarium to diamondback moth larvae. New Zeal. Plant. Prot. 2019, 72, 253–259.
- Benhamou, N.; Brodeur, J. Pre-inoculation of Ri T-DNA transformed cucumber roots with the mycoparasite, Verticillium lecanii, induces host defense reactions against Pythium ultimum infection. Physiol. Mol. Plant. Pathol. 2001, 58, 133–146.
- Hirano, E.; Koike, M.; Aiuchi, D.; Tani, M. Pre-inoculation of cucumber roots with Verticillium lecanii (Lecanicillium muscarium) induces resistance to powdery mildew. Res. Bull. Obihiro Univ. 2008, 29, 82–94.
- Aghdam, S.A.; Fotouhifar, K.B. Introduction of some endophytic fungi of sour cherry trees (Prunus cerasus) in Iran. Rostaniha 2017, 18, 77–94.
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 2007, 53, 802–808.
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 2006, 37, 624–632.
- Bhagyasree, S.; Ghosh, S.; Thippaiah, M.; Rajgopal, N. Survey on natural occurrence of endophytes in maize (Zea mays L.) ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2526–2533.
- Zimowska, B.; Okoń, S.; Becchimanzi, A.; Krol, E.D.; Nicoletti, R. Phylogenetic characterization of Botryosphaeria strains associated with Asphondylia galls on species of Lamiaceae. Diversity 2020, 12, 41.
- Mitina, G.; Kazartsev, I.; Vasileva, A.; Yli-Mattila, T. Multilocus genotyping based species identification of entomopathogenic fungi of the genus Lecanicillium (=Verticillium lecanii sl). J. Basic Microbiol. 2017, 57, 950–961.
- Kouvelis, V.N.; Sialakouma, A.; Typas, M.A. Mitochondrial gene sequences alone or combined with ITS region sequences provide firm molecular criteria for the classification of Lecanicillium species. Mycol. Res. 2008, 112, 829–844.
- Nonaka, K.; Kaifuchi, S.; Ōmura, S.; Masuma, R. Five new Simplicillium species (Cordycipitaceae) from soils in Tokyo, Japan. Mycoscience 2013, 54, 42–53.
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.S.J.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmão, L.F.P.; Souza-Motta, C.M.; et al. Fungal planet description sheets: 716–784. Persoonia 2018, 40, 240–393.
- Anderson, C.M.; McGee, P.A.; Nehl, D.B.; Mensah, R.K. The fungus Lecanicillium lecanii colonises the plant Gossypium hirsutum and the aphid Aphis gossypii. Australas. Mycol. 2007, 26, 65–70.
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop. Prot. 2011, 30, 349–353.
- Lin, Y.; Hussain, M.; Avery, P.B.; Qasim, M.; Fang, D.; Wang, L. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS ONE 2016, 11, e0151844.
- Lin, Y.; Qasim, M.; Hussain, M.; Akutse, K.S.; Avery, P.B.; Dash, C.K.; Wang, L. The herbivore-induced plant volatiles methyl salicylate and menthol positively affect growth and pathogenicity of entomopathogenic fungi. Sci. Rep. 2017, 7, 40494.
- Jackson, D.; Skillman, J.; Vandermeer, J. Indirect biological control of the coffee leaf rust, Hemileia vastatrix, by the entomogenous fungus Lecanicillium lecanii in a complex coffee agroecosystem. Biol. Control. 2012, 61, 89–97.
- Romero, D.; De Vicente, A.; Zeriouh, H.; Cazorla, F.M.; Fernández-Ortuño, D.; Torés, J.A.; Pérez-García, A. Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon. Plant. Pathol. 2007, 56, 976–986.
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128.
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis 2009, 30, 2996–3005.
- Kim, J.J.; Goettel, M.S.; Gillespie, D.R. Potential of Lecanicillium species for dual microbial control of aphids and the cucumber powdery mildew fungus, Sphaerotheca Fuliginea. Biol. Control 2007, 40, 327–332.
- Goettel, M.S.; Koike, M.; Kim, J.J.; Aiuchi, D.; Shinya, R.; Brodeur, J. Potential of Lecanicillium spp. for management of insects, nematodes and plant diseases. J. Invertebr. Pathol. 2008, 98, 256–261.
- Gurulingappa, P.; Mc Gee, P.; Sword, G.A. In vitro and in planta compatibility of insecticides and the endophytic entomopathogen, Lecanicillium lecanii. Mycopathologia 2011, 172, 161–168.
- Ondráčková, E.; Seidenglanz, M.; Šafář, J. Effect of seventeen pesticides on mycelial growth of Akanthomyces, Beauveria, Cordyceps and Purpureocillium strains. Czech. Mycol. 2019, 7, 123–135.
- Cuthbertson, A.G.S.; Blackburn, L.F.; Northing, P.; Luo, W.; Cannon, R.J.C.; Walters, K.F.A. Chemical compatibility testing of the entomopathogenic fungus Lecanicillium muscarium to control Bemisia tabaci in glasshouse environment. Int. J. Environ. Sci. Technol. 2010, 7, 405–409.
- Ali, S.; Zhang, C.; Wang, Z.; Wang, X.M.; Wu, J.H.; Cuthbertson, A.G.; Shao, Z.; Qiu, B.L. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci. Rep. 2017, 7, 46558.
- Kumar, C.S.; Jacob, T.K.; Devasahayam, S.; Thomas, S.; Geethu, C. Multifarious plant growth promotion by an entomopathogenic fungus Lecanicillium psalliotae. Microbiol. Res. 2018, 207, 153–160.
This entry is offline, you can click here to edit this entry!
