Improvements in the growth, yield, and quality of horticultural crops require the development of simply integrated, cost-efficient, and eco-friendly solutions. Hydrogen gas (H2) has been observed to have fertilization effects on soils by influencing rhizospheric microorganisms, resulting in improvements in crop yield and quality. Ample studies have shown that H2 has positive effects on horticultural crops, such as promoting root development, enhancing tolerance against abiotic and biotic stress, prolonging storage life, and improving postharvest quality of fruits, vegetables and cut flowers.
- hydrogen gas
- hydrogen-rich water
- hydrogen nanobubbles
- solid H-storage material
- horticultural crops
- metabolism
1. Introduction
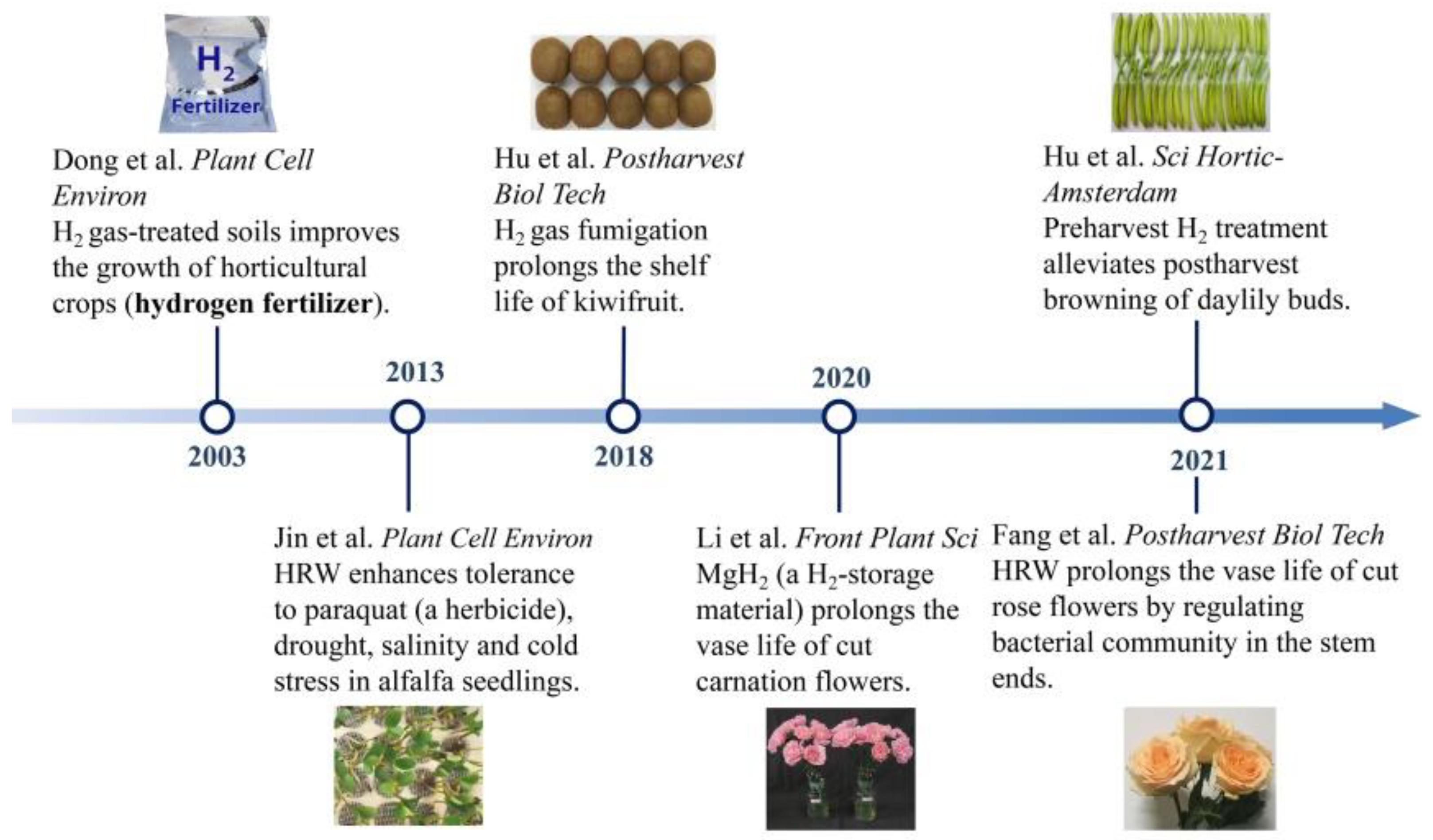
Figure 1. The developing profiles of the application of H2 in horticulture.
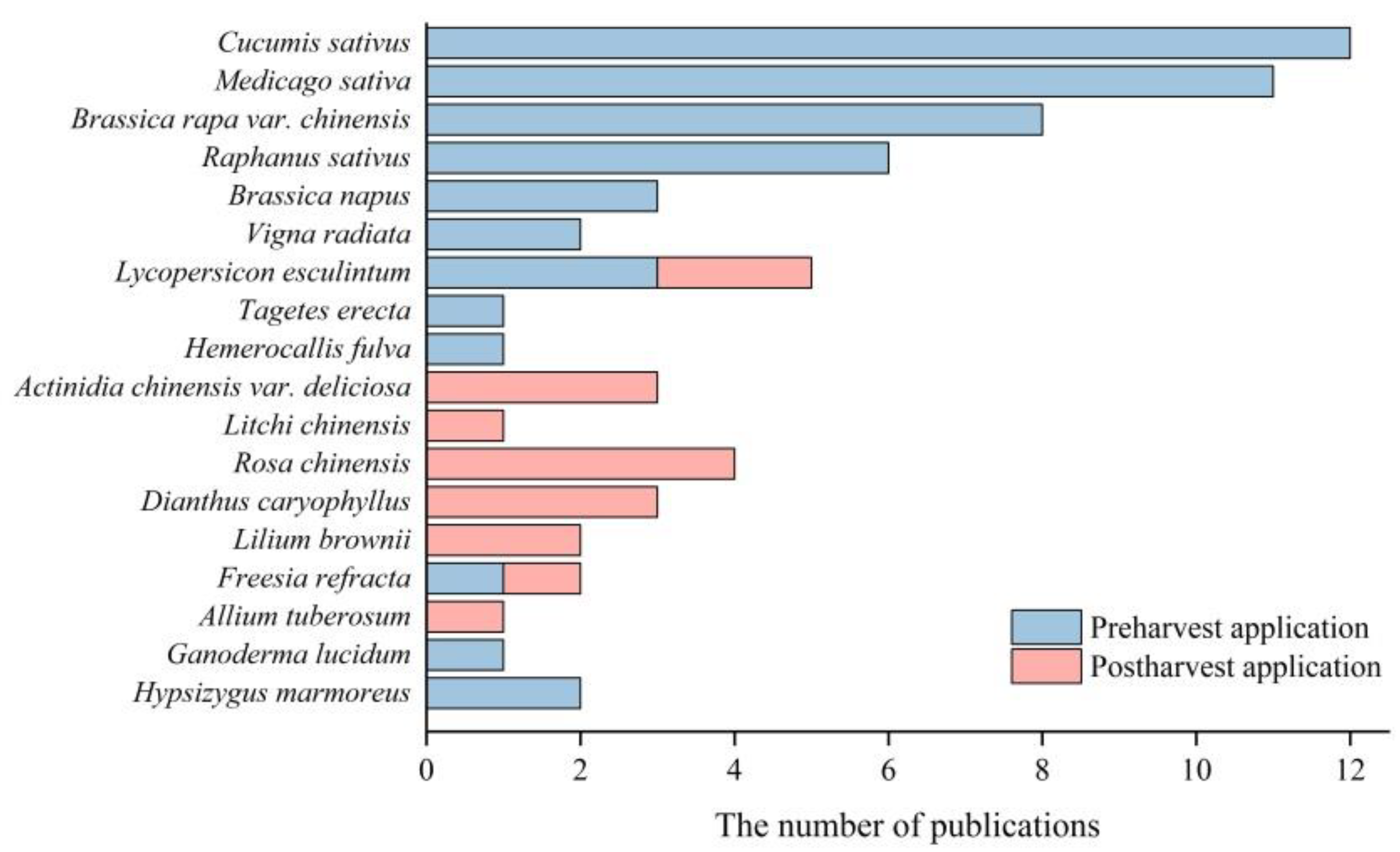
Figure 2. The species of the publications studied on the application of H2 in horticulture.
2. Possible Mechanisms Underlying H2 Responses in Horticultural Crops
2.1. Involved in Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) Metabolism
| Materials | Treatment Stage | H2 Delivery Methods and Treatment | Effective Concentration of H2 | Functions of H2 | Mechanism | Ref. No. |
|---|---|---|---|---|---|---|
| Brassica rapa var. chinensis ‘Dongfang 2′ | Preharvest | 1/4 Hoagland’s nutrient solution with H2 (830 µM); the seedlings were pretreated for 48 h | ~415 µM | Alleviates cadmium toxicity | Regulates NR-dependent NO signaling and enhances antioxidant capacity | [53] |
| 1/4 Hoagland solution with H2 (865 µM); the seedlings were pretreated for 2/3 d (replaced every 12 h) | 865 µM | Reduces cadmium uptake in plant roots | Control of NADPH oxidase encoded by RbohD, which operates upstream of IRT1, and regulates root Cd uptake at both the transcriptional and functional levels | [54] | ||
| Medicago sativa ‘Biaogan’ | Preharvest | HRW (220 µM); the seedlings were pretreated for 12 h | ~110 µM | Enhances tolerance to paraquat | Modulates HO-1 signaling | [11] |
| Alleviates aluminum toxicity | Decreases NO production | [41] | ||||
| HRW (780 µM); the seedlings were pretreated for 12 h | ~390 µM | Induces osmotic stress tolerance | Regulates H2O2 and HO-1 signaling | [38] | ||
| NO-mediated proline accumulation and reestablishment of redox balance | [70] | |||||
| Cucumis sativus ‘Xinchun 4′ | Preharvest | HRW (450 µM); the seedlings were incubated for 2/5 d (changed daily) |
~225 µM | Promotes adventitious rooting | Regulates CO signaling and activates antioxidant system | [34] |
| Regulates NO signaling | [73,74] | |||||
| Induces adventitious rooting under cadmium stress | Decreases oxidative damage, increases osmotic adjustment substance content, and regulates rooting-related enzyme activity | [71] | ||||
| Cucumis sativus ‘Jinyou 35′ | Preharvest | HRW (450 µM); the seeds were soaked for 8 h | 450 µM | Enhances cold tolerance | Enhances antioxidant capacity and slows dehydration rate by improving osmotic adjustment ability | [33] |
| Solanum lycopersicum ‘Baiguoqiangfeng’ | Preharvest | AB@hMSN (10 mg/L); the seedlings were incubated for 2/5 d | ~400 µM | Induces lateral root formation | Modulates NR-dependent NO synthesis, cell cycle regulatory genes, and miRNAs expression | [30] |
| Hypsizygus marmoreus | Preharvest | HRW (1000 µM); the mycelia were cultivated until harvesting | ~250 µM | Increases postharvest quality | Enhances antioxidant defense | [46] |
| Hemerocallis fulva ‘Dawuzui’ | Preharvest | HRW (1.6 µM); irrigation at the stages of bolting, growing and the day prior to the period of harvest |
~0.8 µM | Promotes daylily bud yield and alleviation of bud browning | Decreases ROS level, increases the unsaturated:saturated fatty acid ratio, endogenous H2 and total phenol content, and reduces PAL and PPO activity | [16] |
| Actinidia chinesis ‘Huayou’ | Postharvest | HRW (660 µM); the fruits were soaked for 5 min | ~528 µM | Delays postharvest ripening and senescence | Enhances antioxidant defense | [17] |
| Litchi chinensis ‘Huaizhi’ | Postharvest | HRW (500 µM); the fruits were soaked for 3 min | ~350 µM | Delays the pericarp browning | Induces antioxidant system-related characters | [18] |
| Rosa chinensis ‘Kardinal’; Lilium brownii ‘Manissa’ | Postharvest | HRW (450 µM); cut flowers were incubated for vase period (changed daily) | ~225 µM (Rose); ~45 µM (Lily) |
Improves the vase life and quality | Maintains water balance and membrane stability by reducing stomatal size and oxidative damage | [19] |
| Allium tuberosum | Postharvest | Gas; the leaves were fumigated for storage period (renewed daily) | ~1.2×103 µM | Prolongs the shelf life and maintain storage quality | Increases antioxidant capacity | [21] |
| Dianthus caryophyllus ‘Pink Diamond’ | Postharvest | HNW (~500 µM); cut flowers were incubated for 3 d (changed daily) | ~50 µM | Prolongs the vase life | Reduces ROS accumulation and senescence-associated enzyme activities | [26] |
| Rosa chinensis ‘Carola’ | Postharvest | MgH2 (0.001 g/L); cut flowers were incubated for vase periods (changed daily) | Not shown | Prolongs the vase life | Maintains ROS balance by modulating NO synthesis | [28] |
| Lilium brownii ‘Manissa’ | Postharvest | HRW; cut flowers were incubated for vase period (changed daily) | Not shown (1% saturation HRW) | Prolongs the vase life | Regulates NO signaling and regulates the expression of the photosynthesis-related AtpA | [50] |
| Freesia refracta ‘Red passion’ | Postharvest | HRW (75 µM); cut flowers were pretreated for 12 h | ~0.75 µM | Prolongs the vase life | Improves antioxidant capacity | [51] |
| Eustoma grandiflorum | Postharvest | HRW (780 µM); cut flowers were incubated for vase period (changed daily) | ~78 µM | Prolongs the vase life | Maintains redox homeostasis | [52] |
2.2. Modulation in Sulfur Compounds’ Metabolism
| Materials | Treatment Stage | H2 Delivery Methods and Treatment | Effective Concentration of H2 | Functions of H2 | Mechanism | Ref. No. |
|---|---|---|---|---|---|---|
| Brassica rapa var. chinensis ‘Dongfang 2′ | Preharvest | 1/4 Hoagland’s nutrient solution with H2; the seedlings were incubated for 48 h (replaced every 12 h) after removing cadmium stress | Not shown (50% saturation HRW) | Enhances cadmium tolerance | Reestablishes reduced GSH homeostasis | [39] |
| Medicago sativa ‘Victoria’ | Preharvest | HRW (220 µM); the seedlings were pretreated for 12 h | ~22 µM | Alleviates cadmium toxicity | Reduces cadmium accumulation and reestablishes GSH homeostasis | [15] |
| Expression regulation of genes relevant to sulfur and glutathione metabolism, resulting in enhanced glutathione metabolism and activating antioxidant defense and cadmium chelation | [76] | |||||
| Decreases oxidative damage, enhances sulfur compound metabolic process, and reestablishes nutrient element homeostasis | [77] | |||||
| Alleviates mercury toxicity | Reduces mercury accumulation and reestablishes redox homeostasis (GSH, AsA, and antioxidant enzymes) | [40] | ||||
| Solanum lycopersicum ‘Baiguoqiangfeng’ | Preharvest | HRW (780 µM); the seedlings were incubated for 4 d (changed daily) | ~390 µM | Influences lateral root branching | Promotes γ-ECS-dependent GSH production | [35] |
| Ganoderma lucidum strain HG | Preharvest | HRW (220 µM); added to the medium after 4 days of mycelium culture. | ~11 µM | Regulates morphology, growth, and secondary metabolism | Increases glutathione peroxidase activity under HAc stress | [42] |
| Dianthus caryophyllus ‘Pink Diamond’ | Postharvest | MgH2 (0.1 g/L MgH2 and 0.1 M PBS (pH 3.4); cut flowers were incubated for vase period (changed daily) | ~400 µM | Prolongs the vase life | H2S-mediated reestablishment of redox homeostasis and increased transcript levels of DcbGal and DcGST1 | [25] |
2.3. Involvement in Flavonoids Metabolism
| Materials | Treatment Stage | H2 Delivery Methods and Treatment | Effective Concentration of H2 | Functions of H2 | Mechanism | Ref. No. |
|---|---|---|---|---|---|---|
| Raphanus sativus ‘Qingtou’; R. sativus ‘Yanghua’ |
Preharvest | HRW (220 µM); 1/4 Hoagland’s nutrient solution with H2 (220 µM H2); the seeds were soaked in HRW for 12 h; sprouts were incubated in nutrient solution with H2 for 3 d (replaced every 12 h) under UV-A | ~220 µM | Regulates anthocyanin synthesis under UV-A | Reestablishes ROS homeostasis and regulates anthocyanin biosynthesis-related gene expression | [37] |
| Raphanus. sativus ‘Yanghua’ | Preharvest | HRW (781 µM); the seedlings were incubated for 48/60 h (replaced every 12 h) under UV-A | ~781 µM | Promotes the biosynthesis of anthocyanin under UV-A | Regulates InsP3-dependent calcium signaling | [86] |
| Involved in phytohormones, MAPKs and Ca2+ signaling | [87] | |||||
| HRW (220 µM); the seedlings were incubated for 72 h (replaced every 12 h) under short wavelength light | ~220 µM | Promotes anthocyanin accumulation under short wavelength light | Promotes activities and transcription of anthocyanin biosynthesis-related enzyme (including CHS and UFGT) | [88] | ||
| Medicagosativa ‘Victoria’ | Preharvest | HRW (781 µM); the seedlings were pretreated for 12 h | ~390 µM | Alleviates UV-B-triggered oxidative damage | Regulates (iso)flavonoids metabolism and antioxidant defense | [83] |
2.4. H2 Is Involved in Carbon and Nitrogen Metabolism
| Materials | Treatment Stage | H2 Delivery Methods and Treatment | Effective Concentration of H2 | Functions of H2 | Mechanism | Ref. No. |
|---|---|---|---|---|---|---|
| Cucumis sativus ‘XinJinchun No. 4′ | Preharvest | Hoagland’s nutrient solution with H2 (220 µM H2); the seedlings were pretreated for 7 d (replaced daily) | ~110 µM | Improves heat tolerance | Improves photosynthetic and antioxidant and increases HSP70 content | [32] |
| Brassica rapa var. chinensis ‘Dongfang 2′ | Preharvest | HRW; 1/4 Hoagland’s nutrient solution with H2 (835.1 μM H2); regarding soil cultivation, sprays with HRW (50 mL) at every 12 h for 17 d; for hydroponic solutions, the seedlings were incubated in 1/4 Hoagland solution with H2 for 4 d (replaced every 12 h) with Ca(NO3)2 | ~417 µM | Reduces Ca(NO3)2 toxicity and improves the growth of seedlings | Enhances antioxidant capacities and reestablishes nitrate homeostasis | [44] |
| Cucumis sativus ’Jinyou 35′ | Preharvest | HRW (450 µM); the seeds were soaked for 8 h | ~450 µM | Enhances lower temperature tolerance | Increases the activities of key photosynthetic enzymes and maintains a high level of carbon and nitrogen metabolism | [90] |
| Hypsizygus marmoreus | Preharvest | HRW (800 µM); mycelia were incubated for 5 d (replaced every 12 h) after removal of cadmium stress | ~800 µM | Alleviates salinity and heavy metal toxicity | Activates pyruvate kinase, along with its induced gene expression | [43] |
| Solanum lycopersicum ‘Jiafen No. 2′ | Postharvest | HRW (780 µM); the fruits were soaked for 20 min | ~585 µM | Reduces nitrite accumulation during storage | Inhibits/increases the activity and transcript level of NR/NiR | [47] |
2.5. Modulation of Ion Homeostasis
| Materials | Treatment Stage | H2 Delivery Methods and Treatment | Effective Concentration of H2 | Functions of H2 | Mechanism | Ref. No. |
|---|---|---|---|---|---|---|
| Brassica rapa var. chinensis ‘Dongfang 2’ | Preharvest | 1/4 Hoagland’s nutrient solution with H2; the seedlings were pretreated for 1 d (replaced every 12 h) | Not shown (50% saturation HRW) | Reduces cadmium accumulation | Inhibits the expression of BcIRT1 and BcZIP2, and reduces cadmium absorption | [94,95] |
| Brassica napus ‘Zhongshuang 11′ | Preharvest | Ammonia borane (NH3∙BH3; 2 mg/L); the seedlings were incubated for 3 d (changed daily) under NaCl, PEG, or Cd stress | ~300 µM | Enhances the tolerance against salinity, drought, or cadmium | Decreases cell death rebuilds redox and ion homeostasis, increases proline content, thus reducing cadmium absorption and accumulation | [29] |
| Cucumis sativus ‘Xinchun 4′ | Preharvest | HRW (450 µM); the seedlings incubated for 2/5 d (changed daily) | ~450 µM | Induces adventitious rooting | Regulates the protein and gene expressions of PM H+-ATPase and 14-3-3 mediated by NO. | [96] |
2.6. H2 Is Involved in Phytohormones Signaling
| Materials | Treatment Stage | H2 Delivery Methods and Treatment | Effective Concentration of H2 | Functions of H2 | Mechanism | Ref. No. |
|---|---|---|---|---|---|---|
| Medicagosativa ‘Victoria’ | Preharvest | HRW; the seedlings were irrigated for 7 d before 15-d drought treatment | Not shown (50% saturation HRW) | Induces drought tolerance | Modulates stomatal sensitivity to ABA and Apoplastic pH | [97] |
| Medicagosativa ‘Victoria’ | Preharvest | 1/4 Hoagland’s nutrient solution with H2 (780 µM H2); the seedlings were pretreated for 12 h | ~390 µM | Induces tolerance against osmotic stress | Involved in phytohormone signaling | [98] |
| Cucumis sativus ‘Xinchun 4′ | Preharvest | HRW (680 µM); the seedlings were incubated for 7 d (changed daily) | ~350 µM | Induces adventitious rooting | Ethylene may be the downstream signaling molecule during H2-induced adventitious rooting, and proteins RuBisCo, SBPase, OEE1, TDH, CAPX, and PDI may play important roles | [100] |
| Cucumis sativus ‘Lufeng’ | Preharvest | HRW (220 µM); incubated for 4 d | ~110 µM | Regulates adventitious root development | Regulates HO-1 signaling | [12] |
| Vigna radiata; Cucumis sativus ‘Jinchun 4′; Raphanus sativus ‘Yanghua’ | Preharvest | 1/8 strength Hoagland nutrition solution with H2 (800 µM); the seedlings were incubated for 5 d (replaced every 12 h) | ~480 µM | Promotes elongation of hypocotyls and roots | Increases GA and IAA contents in the hypocotyl and the root | [99] |
| Vigna radiata | Preharvest | HRW; seeds were soaked for 3 d | 100/250 µM | Promotes the growth of shoots and roots | Involved in phytohormone signaling | [31] |
| Freesia refracta | Preharvest | HRW (75 µM); the bulbs were soaked for 6 h; irrigated HRW at every 7–10 d and total 3 times after scape sticking out | ~37.5 µM | Promotes early flowering; increases the number and diameters of florets | Regulates phytohormone and soluble sugar content | [45] |
| Actinidia deliciosa ‘Xuxiang’ | Postharvest | Gas; the fruits were fumigated for 24 h/12 h + 12 h | ~0.2 µM | Prolongs the shelf life | Decreases ethylene biosynthesis | [22] |
| Rosa chinensis ‘Movie star’ | Postharvest | HRW (235 µM); cut flowers were incubated for vase periods (changed daily) | ~2.35 µM | Alleviates postharvest senescence | Inhibits ethylene production and alleviates ethylene signal transduction | [49] |
3. Conclusions and Prospects
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae7110513
