2.1. Adenosine and CD73
Adenosine is a precursor and a breakdown product of an important excitatory neurotransmitter in the CB, ATP, which is tonically released from the type I cell [
6]. Extracellular concentrations of adenosine and ATP are reported to be approximately 20 pmol/CB and 4 pmol/CB respectively, in normoxia and increase by 174% and 147% in mild hypoxia in the rat CB [
7,
8]. A
2A and A
2B-receptor mRNA has been isolated from rat CBs, and immunocytochemical and in situ hybridization techniques have shown that A
2A- and A
2B-receptors are present on the type I cell and A
2A-receptors are expressed on post-synaptic sensory fibers [
9,
10,
11,
12]. In contrast, A
1- and A
3-receptors do not appear to be present in the CB [
9,
12].
CB activation by exogenous adenosine in vivo evokes an acute increase in respiratory frequency, tidal volume and minute ventilation in the rat [
13]. More recent studies have now also confirmed that acute adenosine administration elevates ventilation in humans [
14]. Exogenous adenosine is capable of activating all aspects of the CB chemotransduction cascade, including inhibition of Twik-related acid-sensitive K
+ (TASK) and voltage-gated K
+ channels, elevation of [Ca
2+]
i, promotion of neurotransmitter release and stimulation of chemoafferent fibers [
11,
15,
16,
17].
Endogenous adenosine plays an important role in establishing baseline neurotransmitter release and chemoafferent activity in normoxia [
11,
18]. The non-selective A
2-receptor antagonist 8-(p-Sulfophenyl)theophylline (8-SPT) causes more than 90% depletion in chemoafferent frequency when measured in the ex vivo CB preparation [
18]. This is particularly important given that it is the rise in baseline chemoafferent activity that most likely promotes chronic reflex stimulation and neurogenic hypertension associated with OSA, SH and HF. Initial reports suggest that caffeine (an adenosine receptor antagonist), does not modify the basal chemoafferent frequency in animals following exposure to chronic intermittent hypoxia (CIH), a robust model of OSA [
19]. However, it must be noted that these animals displayed a reduction rather than elevation in baseline nerve activity, which is not characteristic of other studies using CIH. More experiments are definitely warranted to evaluate this further as well as a potential role for adenosine in mediating CB hyperactivity in SH and HF.
Adenosine also has an important role in mediating CB responses to hypoxia [
20,
21,
22] and hypercapnia [
18,
23]. Inhibition of adenosine receptors has a greater impact at low-intensity levels of hypoxia [
8], suggesting that adenosine is important in establishing CB hypoxic sensitivity and/or that adenosine release predominates under milder hypoxic conditions. However, the exact signaling mechanism remains controversial. Both A
2A- and A
2B-receptors are coupled by the G
αs protein, thus a role for adenylyl cyclase (AC) activation and cAMP accumulation appear highly likely [
5,
24]. In the whole CB preparation, A
2B-, but not A
2A-receptor antagonists depress catecholamine secretion in hypoxia [
11,
25]. In these same studies, it was reported that both A
2A- and A
2B-receptor antagonists reduce chemoafferent frequency in hypoxia, leading the authors to conclude that adenosine acts on pre-synaptic A
2B- and post synaptic A
2A-receptors. This is supported by the finding that A
2B- not A
2A-receptor inhibition suppresses type I cell catecholamine secretion and rises in [Ca
2+]
i induced by adenosine in co-cultures with petrosal neurons [
10]. However, in an earlier study, it was observed that A
2A-receptor antagonism almost completely abolished the adenosine-mediated elevation in Ca
2+, an effect that was mimicked by the protein kinase A (PKA) inhibitor H89 [
17]. Other studies have shown that H89 and other PKA inhibitors do not significantly alter the catecholamine release caused by hypoxia [
26]. This has led to the hypothesis that whilst increased adenosine and cAMP are important, they may be acting independently of PKA. Alternative messengers of cAMP include exchange proteins activated by cAMP (EPACs), which when activated, can overcome the inhibition of AC in the CB [
26]. EPAC is suggested to be a key regulator of exocytotic machinery and K
+ channels. Although no direct link between adenosine and EPAC has yet been established, as studies so far have only looked at the link between hypoxia and EPAC, a link is plausible and could be the focus of future investigations. Another target could be the hyperpolarization-activated cation current I
h reported to be activated via an A
2A-receptor and cAMP-dependent mechanism on the post-synaptic site [
27].
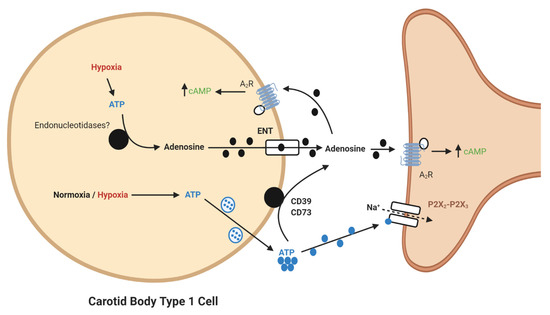
An important consideration is the actual source of adenosine in normoxia and hypoxia. It has been suggested that adenosine may be formed inside type I cells by the breakdown of cAMP and ATP and subsequently released through the bidirectional equilibrative nucleoside transporter (ENT) into the synapse [
7,
8]. This source of adenosine might be expected to increase proportionally during hypoxia as oxidative phosphorylation decreases and cAMP increases. It is therefore somewhat surprising that the functional impact of adenosine is more apparent at low- rather than high-intensity hypoxia [
8]. Furthermore, our own experiments found that pharmacological inhibition of the ENT did not modify chemoafferent frequency in either normoxia or hypoxia [
22]. This is possibly suggestive of an alternative source of adenosine.
It is now apparent that adenosine can be generated from extracellular ATP (). In the CB, a significant synaptic ATP concentration is due to the tonic vesicular neurosecretion from the type I cell [
28] and also through ATP release from type II cells via pannexin-1 channels [
29]. There may also be ATP release from red blood cells, although this remains to be clarified in the CB circulation. CD73 (ecto-5′-nucleotidase) catalyzes the formation of adenosine from AMP, following initial breakdown of ATP and adenosine diphosphate (ADP) by CD39 (ecto-nucleosidetriphosphate diphosphohydrolase) [
30]. RNA and protein expression of CD73 have now been identified in the CB with the majority being localized to the type I cell [
31]. Inhibiting CD73 with α,β-Methylene-ADP (AOPCP) decreases the total pool of extracellular adenosine in the CB [
7]. Furthermore, similar pharmacological inhibition of CD73 causes a dramatic reduction in basal chemoafferent activity and blunts the CB response to hypoxia in vitro and the cardiovascular-respiratory response to hypoxia in vivo [
22]. Given the high concentrations of AOPCP used in these studies, there is still a need to validate these findings using genetic models or with lower doses of more selective CD73 inhibitors that are becoming available. Consistent with a hypoxia-inducible factor-1 (HIF-1)-dependent elevation of CD73 in other tissues, including hypoxic tumors [
32], CB CD73 expression increases in response to chronic hypoxia along with A
2B-receptors [
10,
33]. Interestingly, a recent study shows that CD73 expression is elevated in the aged CB, despite an overall reduction in chemoreceptor function [
34]. However, a role for CD73 in mediating CB hyperactivity in CIH, SH or HF remains to be elucidated.
Figure 1. Schematic illustration of ecto-5′-nucleotidase (CD73)-mediated adenosine generation and signaling in the carotid body (CB). During normoxia/hypoxia, ATP released as a neurotransmitter can be converted to adenosine by the action of ecto-nucleosidetriphosphate diphosphohydrolase (CD39) and CD73. Alternatively, ATP can be converted to adenosine in the type I cell and released via the equilibrative nucleoside transporter (ENT). Adenosine binds to A2-receptors on the pre- and post-synaptic membrane to increase baseline activity and overall hypoxic sensitivity. Filled lines denote purinergic signaling, dashed lines denote ion flow.
2.2. Adrenaline
Although adenosine is regarded as the major positive regulator of cAMP in the CB, there are other substances that may elevate cAMP. One of these is adrenaline. Adrenaline binds to β-adrenoceptors, most commonly the β
1 and β
2 subtypes which are coupled to the G
αs subunit, resulting in cAMP elevation in heart and other tissue [
35]. Although there is substantial evidence that adrenaline stimulates breathing [
36,
37], the direct effect on the CB is less clear. There are reports of exogenous adrenaline administration in vivo causing both increased and decreased CB chemoreceptor discharge [
36,
38,
39]. The effect might be dependent on the dose/concentration used, whilst physiological concentrations selectively act on β-adrenoceptors, supra-physiological levels could act on D
2-receptors leading to inhibition. This is supported in our own work where we identified a stimulatory effect at 10 nM but an inhibitory action at higher concentrations, as evidenced by a marked reduction in chemoafferent frequency [
37,
40].
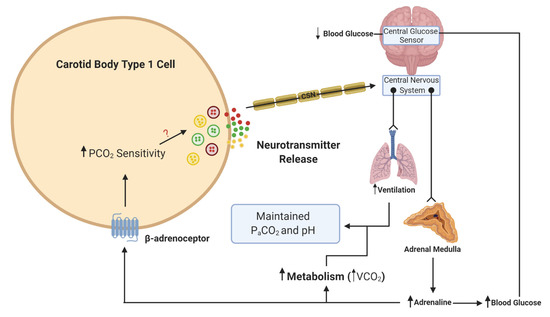
What is more important to consider is the physiological relevance of endogenous adrenaline. Our own understanding of the effects of endogenous adrenaline have been developed through the study of the counter-regulatory response to hypoglycemia. During hypoglycemia, adrenaline levels rise [
41], and hepatic glucose release increases to counter this fall. Multiple studies have now provided evidence of activation of the CB in response to hypoglycemia in animals [
42,
43] and humans [
44,
45]. This is essential not only to further augment hepatic glucose release but also to increase ventilation to match the concurrent rise in metabolic rate, initiated by adrenaline [
43,
46] (). In these circumstances, a heightened CB CO
2 sensitivity allows for increased chemoreceptor discharge and ventilation in the absence of any change in arterial blood gases. Recent evidence has shown that the increase in minute ventilation and CO
2 sensitivity during hypoglycemia was blunted by propranolol treatment, removal of the adrenal gland and section of the carotid sinus nerve (CSN) [
37]. This is consistent with the view that it is adrenaline that activates the CB during hypoglycemia, via β-adrenoceptors. Key experiments are now needed to see if this is also evident in humans. Perhaps surprisingly, no studies have yet looked at a potential role for adrenaline in causing CB hyperactivity in OSA or HF, both of which are associated with a chronic rise in plasma catecholamines. Chronic isoprenaline delivered via osmotic mini-pump does elevate baseline breathing and responses to hypoxia and hypercapnia [
40], but as yet, the relevance of this model to pathology remains uncertain and studies in animals and/or humans with HF and CIH are required [
47]. Moreover, there is hardly any indication of how adrenaline acts to augment CB chemosensitivity (even the specific receptor subtypes) and there is limited evidence of the impact of beta-blockers on CB function. Key experiments are needed to establish if chronic beta-blocker treatment (which are widely prescribed for heart failure, hypertension and arrhythmia) dampens CB function such that there is increased vulnerability to hypoxic and hypoglycemic exposure. As such, we are just beginning to grasp the importance of adrenaline in mediating CB function in health and disease.
Figure 2. Schematic illustration of adrenaline activation of the carotid body (CB) during hypoglycemia. When blood glucose decreases, this is sensed in the brainstem and leads to a reflex increase in adrenaline release from the adrenal medulla. β-adrenoceptors in type 1 cells will be activated by adrenaline. This activation will lead to an increase in CO2 sensitivity, neurotransmitter release and an increase in ventilation. The elevation in ventilation matches the increase in metabolic rate and CO2 generation (VCO2), such that the overall partial pressure of arterial CO2 (PaCO2) and pH remain constant. The ? denotes that the signaling mechanism linking increased CO2 sensitivity with enhanced neurotransmitter release is still unknown. Lines with arrows denote signaling pathways. Lines with circles and chevrons denote efferent neurons.
2.3. Lactate and Olfr78
Lactate signaling through the GPCR Olfr78 (G
αs) has gained significant attention based on the finding that Olfr78 mRNA is highly expressed in the murine CB and its global deletion completely abolishes the hypoxic ventilatory response (HVR) [
48]. This was coupled with a complete depression of CB chemoafferent activity during hypoxia. Indeed, it is logical that both intracellular and systemic lactate levels increase during hypoxia after partial or complete termination of oxidative phosphorylation in the CB and other tissues, thereby providing a stimulus for Olfr78. The work has now been strengthened by evidence from another laboratory demonstrating a similar depression in the HVR in a different mouse strain [
49]. However, this was in spite of severe hypoxia failing to depress the elevation in type I cell [Ca
2+]
i in Olfr78
−/−, raising questions over the exact location of the Olfr78 signaling within the CB or chemoreflex pathway. Furthermore, this same study observed similar elevations in chemoafferent nerve activity induced by lactate in Olfr78
−/− and wildtype (WT) CBs, suggesting that another ligand, and not lactate, activates Olfr78 during hypoxia. In contrast to these studies, it has also been reported that that Olfr78
−/− mice maintain a robust HVR and retain type I cell sensitivity to hypoxia [
50]. Clearly there is a need to reconcile these findings and in particular to study lactate and Olfr78 in higher species, including humans, which have a larger chemoafferent response to hypoxia and are less susceptible to respiratory alkalosis. In our own studies, we observed that after prolonged exposure to glucose deprivation, which eventually caused chemoafferent excitation, 5 mM lactate acted to suppress rather than excite chemoafferent frequency [
51]. Despite being in a different experimental setting, we propose that lactate may have an additional role as an alternative energy source, independent of Olfr78, similar to that seen in the central nervous system (CNS) and peripheral nerves [
52,
53].
2.4. Dopamine and Noradrenaline
The CB contains a large amount of stored catecholamines (CAs) in dense core vesicles, and for its mass, the overall CA content is equivalent only to that seen in adrenal medullary tissue [
54]. The enzyme tyrosine hydroxylase (TH), that initiates the synthesis of CAs from tyrosine, has now emerged as a well-established type I cell marker [
55]. Dopamine (DA) is the most abundant CA in type I cells, forming more than 50% of the overall CA content [
54,
56]. There is some evidence of dopamine beta-hydroxylase expression in type I cells, consistent with small amounts of noradrenaline (NA) [
57]. Interestingly, dopamine beta-hydroxylase is augmented in CBs of SH rats, possibly indicative of a more pronounced role of NA as a transmitter in pathology [
58]. Recently, it has been demonstrated that type I cells contain significant amounts of vesicular monoamine transporter 1 (VMAT1), the enzyme most likely responsible for incorporation of DA and NA into secretory vesicles [
59,
60]. This process may also be highly sensitive to levels of biotin, a vitamin and coenzyme known to accumulate in type I cells [
59]. Future experiments may well evaluate how these enzymes are altered in CB-mediated cardiovascular and metabolic disease.
DA is released from type I cells in abundance during hypoxia [
61,
62], but its action seems to be autoinhibitory, providing the CB with a degree of inhibitory feedback control via D
2-receptors [
63,
64,
65,
66]. Indeed, it has been suggested that the balance between dopamine (via G
αi-coupled D
2-receptors) and adenosine (via G
αs-coupled A
2B-receptors) is critical in determining the overall cAMP level and excitability within the type I cell [
25]. The increase in [Ca
2+]
i in rat type I cells in response to hypoxia can be attenuated by D
2-receptor agonists [
67]. D
2-receptors have now also been positively confirmed in the human CB [
68] and DA infusion to depress CB function is commonly used to estimate CB contribution to pathophysiological reflexes in humans [
69,
70,
71]. Whether or not DA completely silences the CB chemoafferent discharge in humans is unknown. Exogenous DA does indeed reduce (but not abolish) the HVR in humans, but there is some considerable variability between individuals and not all of the observed effects may be solely attributable to CB inhibition [
72,
73]. In mice with global deficiency of D
2-receptors, whilst type I cell neurotransmitter release was enhanced in response to hypoxia, the chemoafferent activity was reduced and the HVR was unchanged [
74]. In rats, the HVR can be enhanced rather than depressed, by systemic inhibition of the monoamine oxidase enzyme, which is likely to have increased free DA [
75]. These studies are suggestive of additional locations and possible excitatory functions of D
2-receptors within the CB and/or chemoreflex pathway other than the type I cell in rodents. Any excitatory action does not however seem to involve cAMP sensitive hyperpolarization-activated currents (I
h), as evidenced by DA causing a reduction in this post-synaptic current through a D
2-receptor-mediated mechanism [
27].
Reverse transcription polymerase chain reaction (RT-PCR) analysis of short- and long-term hypoxic rats’ CBs showed a time-dependent increase in the expression of TH and D
2-receptor genes [
76,
77]. After 48 hours of hypoxia, D
2-receptor mRNA levels decreased, but after 7 days, the expression of D
2-receptor increased significantly [
76]. The alteration in D
2-receptor expression has been hypothesized to cause a change in DA signaling in the CB which contributes to the changes in ventilatory adaptation observed with long-term hypoxia associated with chronic obstructive pulmonary disease (COPD) or HF [
78]. A role for DA in establishing CB hyperactivity in response to CIH has recently been investigated [
79]. In this study, CIH augmented CB DA content, CB catecholamine release and arterial blood pressure, but not the HVR. Moreover, the D
2-receptor antagonist domperidone reversed the elevation in blood pressure and the excessive catecholamine release during hypoxia in CBs isolated from CIH animals. This again goes against the idea that DA is simply an inhibitory neurotransmitter and opens up the possibility that it has an excitatory function in the CB in certain pathological conditions. In this instance, the authors do also point out that some of the effects of domperidone may have been due to changes in either systemic or local CB blood flow.
NA makes up approximately 15–40% of the total catecholamine content of the CB (varying in different species) and is located in the dense core secretory vesicles in type I cells [
80,
81]. In acute hypoxia, NA is secreted from the type I cells into the extracellular space in proportion to the stimulus intensity [
80]. Release of NA from sympathetic terminals and uptake of NA from the circulation also contributes to the total extracellular NA content in the CB. Despite its concentration being significantly lower than that of DA, NA still has an important functional role in CB chemoreception. In a study performed in anaesthetized dogs in the 1970s, it was first observed that infusion of NA into the carotid artery produced a burst of chemoafferent excitation followed by a more sustained inhibition [
82]. Similar intracarotid infusions of α
2-adrenoceptor agonists produced chemoafferent inhibition and blunted the response to hypoxia in anaesthetized cats [
83]. Intracarotid injection of NA has been shown to inhibit ventilation in goats, a response that was attenuated by both α-adrenoceptor and D
2-receptor antagonists [
84]. Sectioning of sympathetic nerves innervating the CB in cats was shown to have no effect on baseline chemoafferent activity but did augment the frequency during hypoxia [
85]. Direct application of NA to the whole ex vivo CB or dissociated type I cells blunts neurotransmitter release, Ca
2+ currents and the rise in cAMP during hypoxia, effects which are again dependent on α
2-adrenoceptor activation [
86,
87]. Thus, there is a strong body of evidence to suggest that endogenous NA released from sympathetic nerves and type I cells promotes inhibitory feedback via G
αi-coupled α
2-adrenoceptors. In contrast, we have seen that venous infusion of NA in anaesthetized rats elevates ventilation that is partially blocked by section of the CSN [
40]. It is possible that this method of administration produced a more sustained/intense vasoconstriction to arterioles supplying the CB, leading to a significant reduction in blood flow and local hypoxia. Alternatively, wide-spread delivery of NA could promote the release of intermediate substances into the systemic circulation that act indirectly on the CB to evoke chemostimulation. Clearly, there is a need to unify these contradictory findings. The impact of sympathetic stimulation on CB blood flow requires more investigation and especially in conditions where there is a known increase in sympathetic tone in other vascular beds. Furthermore, it is currently unknown what the chronic effect of elevated plasma NA could be on CB blood flow and function, especially common in conditions such as OSA, SH, HF and pheochromocytoma.