Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
Sulfamethoxazole (SMX) is a frequently used antibiotic for the treatment of urinary tract, respiratory, and intestinal infections and as a supplement in livestock or fishery farming to boost production.
- sulfamethoxazole
1. Sources of Sulfamethoxazole in Water and Their Environmental Impacts
There are various point and non-point sources of SMX in rivers and other water resources. The major sources of SMX (antibiotic) into water are illustrated graphically in Figure 1. The primary sources of this molecule into the water resources can be classified into five different groups: (i) effluent wastes from hospitals, (ii) effluent waste from pharmaceutical industries, (iii) effluent waste from the water treatment plant, (iv) effluent waste from aquaculture system and livestock farming, and (v) effluent and leachates from sanitary landfills [1]. The concentration of SMX in the river stream and water reportedly varies from ng/L to mg/L [1]. The SMX from the aquaculture system and livestock farming mostly come into the river stream through the disposal of wastewater generated from the farms directly into the river. The concentration of SMX from aquaculture systems (fish and shrimp) and livestock farms (pig and poultry) reportedly varies from 10 μg/L to 7 mg/L as reported in the water samples collected from shrimp ponds and canals nearby the livestock farming in Vietnam [2][3]. Untreated sewage is another source of SMX into the water as, during the heavy rainfall, untreated sewage, due to having limited hydraulic capacity, finds its way into the freshwater line. The concentration of SMX from the sewage discharge reportedly varies from ng/L up to 60 μg/L, as indicated by the data of the analysis of water samples from the sewage line discharge and freshwater streams within its nearby areas [4]. Apart from that, the presence of SMX in the effluent of wastewater treatment plants was also observed in the water samples, as reported in studies conducted in different countries [5][6]. The concentration of this compound in the effluent of sewage treatment plants reportedly varies from 226 to 3000 ng/L as per the data obtained from different studies conducted in China, Vietnam, and the Philippines [3][7][8]. Leachate from landfills is another major source of SMX discharge into the surface and groundwater. However, very little information and understanding of the mechanism is available regarding the fate and transport of this compound from the landfill sites. The concentration of SMX in the leachates reportedly varies from 6.4 to 8488 ng/L based on the data gathered from the studies conducted in the landfill sites of China and Singapore [9][10]. Moreover, the effluent waste from the pharmaceutical industries and hospital wastes are categorized as one of the direct sources of SMX and other antibiotics into the water. Previous studies carried out in the cities of China and Vietnam indicated the presence of SMX and other antibiotics in wastewater generated from hospitals and pharmaceutical industries [11][12]. The typical concentration of SMX in the wastewater generated from the pharmaceutical industry and hospitals reportedly varied from 320–2910 ng/L based on the data of the studies done earlier in China and Vietnam [11][13].
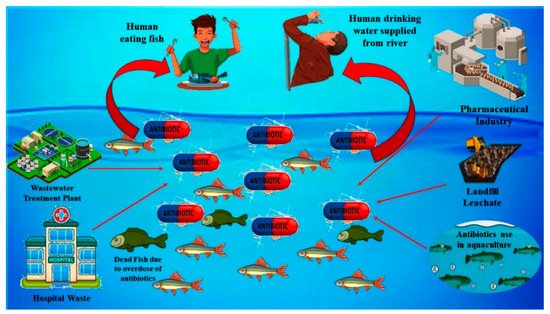
Figure 1. Graphical illustration of various point and non-point sources of SMX into the rivers and water resources.
2. Photochemistry of Sulfamethoxazole in Water
Sulfamethoxazole (SMX) and tetracycline are widely used antibiotics, which are mostly used for the treatment of human beings and animals [14]. These antibiotics are usually detected in wastewater, surface water, and groundwater in the detection range of ng L−1 to μg L−1 [15][16]. Specifically, sulfamethoxazole (SMX), which comes under the chemical class of sulfonamide compounds, is an antimicrobial drug with broad-spectrum activity against gram-positive and negative bacteria [17]. The pharmaceutical waste, of which the antibiotic is a part, is usually hydrolyzed in water. The understanding of the degree and behavior of hydrolysis of antibiotics is of prime concern to understand their stability and non-biodegradability in the environment [18]. Sulfamethoxazole possesses good chemical stability in the environment, which allows it to resist metabolic processes and natural degradation [19]. The SMX usually absorbs the incident radiation light in the wavelength range of 250 to 300 nm, which also varies with respect to the change in the surface charge density on the SMX as the pH of the aqueous solution varies from acidic to alkaline [20]. The absorbance range reportedly shifted towards a higher wavelength side (up to 300 nm) as the pH of the aqueous solution was reduced to 1 from 10 (Figure 2) [20]. The pH of the solution plays a key role in the photocatalytic decomposition of SMX in water, as the SMX degradation rate reportedly was decreased with the increase in the pH value of the aqueous solution, as shown in Figure 3a [21]. The SMX mostly exists in the anionic form if pH > 5.6, and in the neutral form if pH lies between 1.85–5.6 (Figure 3b), as the pKa1 and pKa2 values of SMX was reported to be about 1.85 and 5.60, respectively [21][20]. The photolytic degradation of SMX is very difficult as it cannot be completely mineralized during oxidation [21]. Previous studies elucidated the photocatalytic oxidation mechanism of SMX and the formation of intermediatory transformation products (TPs) [21][20]. The detailed mechanism of SMX photodegradation in the presence of reactive oxygen species (ROS), i.e., •OH radical is shown in Figure 4. It can be observed from the figure that SMX formed its isomerization intermediatory product and is denoted by P 254. Subsequently, with the addition of •OH radical, the SMX is degraded to an intermediatory product denoted by 270 c. Thereafter, the addition of •֗OH radical to the transformation product (TP) defined as 270 c led to the demethylation process, which resulted in the formation of a new TP denoted by P 288. Subsequently, the breakage of the N–O bond and the removal of the carboxylic group from P 288 lead to the formation of another TP denoted by P 246. Additionally, the release of N–O and C–C bonds from P 288 formed another intermediatory product or TP denoted by P 198. The cleavage of the S–N bond from the SMX structure was reportedly occurred after reacting with an •OH free radical following the binding of N to an H atom, which leads to the formation of another TP called 3-amino 5-methylisoxazole denoted as P 99 [20]. The isomerized product of SMX is denoted by P 254, which, after reacting with •OH free radical, leads to the scission of S–N bond from P 254 that leads to the formation of a TP denoted by P 174. The P 174 on further oxidation with ROS leads to the formation of two new TPs denoted as P 158 and P 95, respectively, following the loss of C–N and C–S bonds.
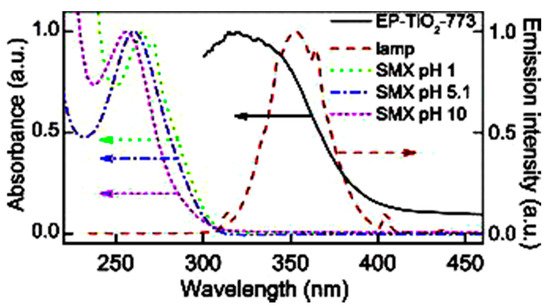
Figure 2. Variations in the wavelength absorbance by SMX with the change in the pH of the aqueous solution (reproduced from [20], Copyright Year 2015, Journal of Hazardous Material @ Elsevier with the permission order number: 5114720328428) (EP-TiO2: Expanded perlite coated TiO2 particles).
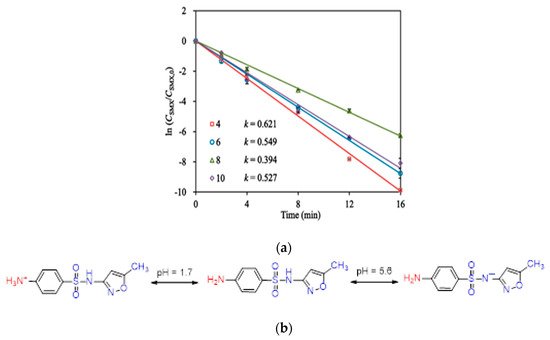
Figure 3. (a) Variations in the photocatalytic degradation of SMX concerning change in the pH of the aqueous solution (Reproduced from [22] (with the permission order number: 5114291052991); (b) charge variation in the structure of SMX with respect to the change in pH from acidic to alkaline in the aqueous solution (adapted from [20], Copyright Year 2015, Journal of Hazardous Material @ Elsevier with the permission order number: 5114720328428).
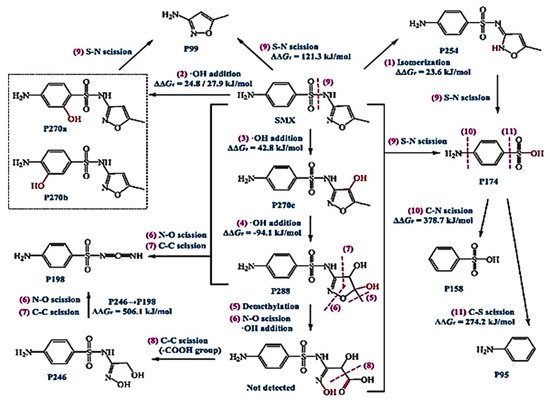
Figure 4. Mechanism of photolytic oxidation/reduction of SMX and the formation of various transformative products (TPs) during its photolysis (reproduced from [21] Copyright Year 2019, Chemical Engineering Journal @Elsevier with permission License number: 5114291052991© Elsevier).
This entry is adapted from the peer-reviewed paper 10.3390/toxics9110313
References
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764.
- Le, T.X.; Munekage, Y. Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Mar. Pollut. Bull. 2004, 49, 922–929.
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati; Nakada, N.; Murata, A.; Suzuki, T.; Suzuki, S.; et al. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013, 452–453, 108–115.
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.H.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174.
- Sim, W.J.; Lee, J.W.; Lee, E.S.; Shin, S.K.; Hwang, S.R.; Oh, J.E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 2011, 82, 179–186.
- Kim, J.-P.; Jin, D.R.; Lee, W.; Chae, M.; Park, J. Occurrence and Removal of Veterinary Antibiotics in Livestock Wastewater Treatment Plants, South Korea. Processes 2020, 8, 720.
- Lien, L.T.Q.; Hoa, N.Q.; Chuc, N.T.K.; Thoa, N.T.M.; Phuc, H.D.; Diwan, V.; Dat, N.T.; Tamhankar, A.J.; Lundborg, C.S. Antibiotics in wastewater of a rural and an urban hospital before and after wastewater treatment, and the relationship with antibiotic use-a one year study from Vietnam. Int. J. Environ. Res. Public Health 2016, 13, 588.
- Jiang, H.; Zhang, D.; Xiao, S.; Geng, C.; Zhang, X. Occurrence and sources of antibiotics and their metabolites in river water, WWTPs, and swine wastewater in Jiulongjiang River basin, south China. Environ. Sci. Pollut. Res. 2013, 20, 9075–9083.
- Wu, D.; Huang, Z.; Yang, K.; Graham, D.; Xie, B. Relationships between Antibiotics and Antibiotic Resistance Gene Levels in Municipal Solid Waste Leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128.
- Shi, Y.; Liu, J.; Zhuo, L.; Yan, X. Antibiotics in wastewater from multiple sources and surface water of the Yangtze River in Chongqing in China. Environ. Monit. Assess. 2020, 192, 1–13.
- City, M.; Vo, T.; Bui, X.; Cao, N.; Luu, V. Investigation of antibiotics in health care wastewater in Ho Chi. Environ. Monit. Assess. 2016, 188, 1–9.
- Anh, H.; Ha, N.; Tung, H.; Thuong, T.; Viet, H.; Pham, V.C.; Berg, M.; Giger, W.; Alder, A.C. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam. Chemosphere 2008, 72, 968–973.
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400.
- Avisar, D.; Lester, Y.; Ronen, D. Sulfamethoxazole contamination of a deep phreatic aquifer. Sci. Total Environ. 2009, 407, 4278–4282.
- Çalişkan, E.; Göktürk, S. Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. Sep. Sci. Technol. 2010, 45, 244–255.
- Dirany, A.; Efremova Aaron, S.; Oturan, N.; Sirés, I.; Oturan, M.A.; Aaron, J.J. Study of the toxicity of sulfamethoxazole and its degradation products in water by a Thaibioluminescence method during application of the electro-Fenton treatment. Anal. Bioanal. Chem. 2011, 400, 353–360.
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553.
- Białk-bieli, A.; Stolte, S.; Matzke, M.; Fabia, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of sulphonamides in aqueous solutions. J. Hazard. Mater. 2012, 222, 264–274.
- Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989.
- Długosz, M.; Zmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic degradation of sulfamethoxazole in aqueous solution using a floating TiO2-expanded perlite photocatalyst. J. Hazard. Mater. 2015, 298, 146–153.
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms. Chem. Eng. J. 2019, 359, 1527–1536.
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of sulfamethoxazole from water via activation of persulfate by Fe3 including mechanism of radical and nonradical process. Chem. Eng. J. 2019, 375, 122004.
This entry is offline, you can click here to edit this entry!
