Muscle fatigue (MF) declines the capacity of muscles to complete a task over time at a constant load. MF is usually short-lasting, reversible, and is experienced as a feeling of tiredness or lack of energy. The leading causes of short-lasting fatigue are related to overtraining, undertraining/deconditioning, or physical injury. Conversely, MF can be persistent and more serious when associated with pathological states or following chronic exposure to certain medication or toxic composites. In conjunction with chronic fatigue, the muscle feels floppy, and the force generated by muscles is always low, causing the individual to feel frail constantly. The leading cause underpinning the development of chronic fatigue is related to muscle wasting mediated by aging, immobilization, insulin resistance (through high-fat dietary intake or pharmacologically mediated Peroxisome Proliferator-Activated Receptor (PPAR) agonism), diseases associated with systemic inflammation (arthritis, sepsis, infections, trauma, cardiovascular and respiratory disorders (heart failure, chronic obstructive pulmonary disease (COPD))), chronic kidney failure, muscle dystrophies, muscle myopathies, multiple sclerosis, and, more recently, coronavirus disease 2019 (COVID-19). The primary outcome of displaying chronic muscle fatigue is a poor quality of life.
- neuroinflammation
- skeletal muscle
- atrophy
- muscle function
- fatigue
1. Muscle Architecture
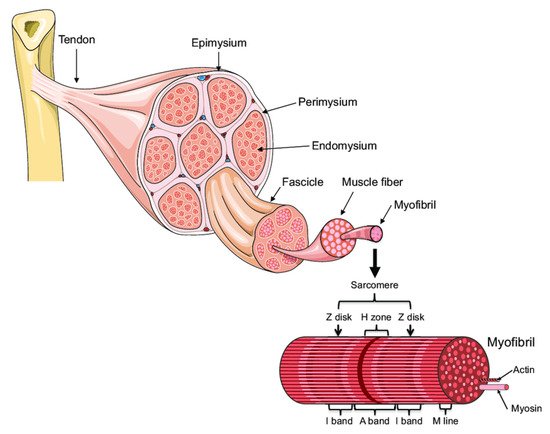
2. Muscle Fatigue
- (1)
-
temporary due to strenuous physical activities and is caused by accumulation in the intracellular space of working muscles with intermediary energy metabolism waste (e.g., lactate) or depletion of their energy-rich compounds (e.g., muscle glycogen store).
- (2)
-
chronic due to either:
- (i)
-
muscle atrophies (muscles waste away) due to immobilization, also called disuse atrophy, the presence of chronic inflammation in cardiovascular and respiratory disorders (e.g., heart failure, chronic obstructive pulmonary disease (COPD)), trauma, critical illness, medication (PPAR agonism),
- (ii)
-
muscle atrophy with aging (sarcopenia), or
- (iii)
-
neurogenic muscle atrophy due to obstructions or interference with different stages of nerve signal propagation from CNS to motor neuron plate due to disease or spinal injury. Depending upon location, it can be divided into central and peripheral [3]. Central fatigue is initiated at the central nervous system (CNS), such as in multiple sclerosis, thereby decreasing the neural drive to the muscle [4][5]. In contrast, peripheral fatigue is generated by changes at or distal to the neuromuscular junction such as in (a) autoimmune diseases caused by abnormal autoimmune reactions targeting neuromuscular synaptic proteins, as in Graves disease, Guillain-Barré syndrome, and myasthenia gravis, or (b) muscular dystrophies (MDs) due to genetic defects. MDs are progressive and debilitating diseases characterized by muscle wasting and progressive weakness.
3. Reduction of Muscle Mass and Function
The main underlying factor behind the loss of muscle mass, malnutrition, and negative nitrogen balance is an increase in skeletal muscle protein degradation. This occurs on a background of inflammatory responses to trauma or infection, increased circulating cytokine, glucagon, epinephrine, and glucocorticoid treatment, hyperglycemia-mediated secondary infections, and induction of muscle insulin resistance. The immobilization is also an important factor triggering the preferential myosin loss, atrophy, and loss of specific force in fast- and slow-twitch muscle fibers with the loss of strength, especially in the quadriceps and extensors [6]. The critical implications of muscle protein loss extend to poor clinical outcomes such as wound healing, decreased ambulation, and increased risk of thromboembolic complications. There is also evidence that trauma and sepsis can lead to pulmonary complications due to a catabolic response in the respiratory muscles [7], extending to peripheral skeletal muscles [8].
Although the amount of protein that is degraded in healthy subjects of a given age equals typically the amount of protein synthesized, the whole-body protein turnover (protein synthesis + protein degradation) decreases gradually with ageing after peaking through puberty.
4. Major Molecular Mechanisms of Underlying Muscle Wasting
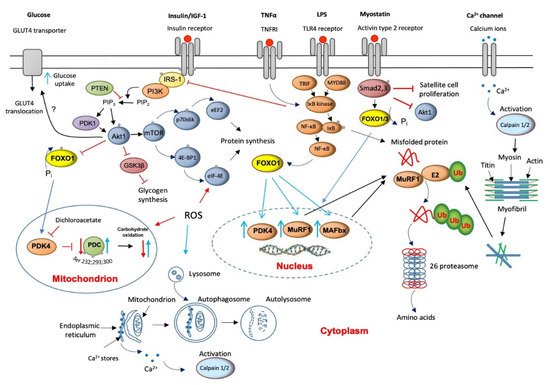
5. The Role of Lysosomal Autophagy in Muscle Protein Breakdown
6. Muscle Calpains’ Expression Is Increased in Muscle Wasting
7. Muscle Myostatin Expression Is Increased in Muscle Wasting
8. Apoptosis Is Increased in Muscle Wasting
9. Critical Illness Is Associated with Muscle Insulin Resistance
10. ROS Involvement in Muscle Wasting
11. Muscle Wasting with Ageing Sarcopenia
12. Muscle Wasting with Chronic Chemical Exposure
13. Muscle Fatigue Associated with Neurogenic Muscle Atrophy Induced by Viruses
A classic example of a disease associated with chronic muscle fatigue is multiple sclerosis. Here, the suppressor function of regulatory T cells (Tregs), which have a role in regulating or suppressing other cells of the immune system, thereby controlling the immune response to self and foreign particles (antigens), is impaired for vague reasons. We recently provided evidence that supports a novel mechanism underlying diminished Treg function in multiple sclerosis. Thus, infections that activate the toll-like receptor 2 (TLR2) in vivo (specifically through TLR1/2 heterodimers) could shift the ratio between the Treg and the HIV inhibitor T helper 17 (Th17) cells’ balance toward a pro-inflammatory state in multiple sclerosis, thereby promoting disease activity and progression [48].
COVID-19 is a recent viral infection that has spread worldwide and has been identified to affect multiple organ systems, including the nervous system. There is now a mounting body of evidence to suggest the existence of a long-term, post-Covid muscle fatigue syndrome even after mild cases of viral infections. There is, so far, no evident description of the underlying pathology. Muscle deconditioning, immune- or virus-mediated neuropathy, and exercise hyperventilation have been hypothesized to play an essential role in developing debilitating symptoms [49]. Nevertheless, due to the presence of robust and durable systemic inflammatory responses to the viral load (such as the formation of ROS and NO by immune cells during chronic inflammation) in conjunction with a lengthy bed immobilization and medication initially intended to dampen the immune responses, which otherwise also stimulates muscle atrophy (e.g., the steroid dexamethasone), it may not be, therefore, surprising that these factors could contribute to accelerating the energy-independent proteolytic activation of protein degradation via calcium (calpains) and TNF-α (E3-ligases) along with the dexamethasone-induced upregulation of myostatin [50]. Collectively, these outcomes might explain the initiation of muscle atrophy and muscle fatigue.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222111587
References
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629.
- Bonetto, A.; Bonewald, L.F. Bone and muscle. In Basic and Applied Bone Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 317–332.
- Wan, J.J.; Qin, Z.; Wang, P.Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384.
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789.
- Bigland-Ritchie, B.; Jones, D.A.; Hosking, G.P.; Edwards, R.H. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin. Sci. Mol. Med. 1978, 54, 609–614.
- Alkner, B.A.; Tesch, P.A. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur. J. Appl. Physiol. 2004, 93, 294–305.
- Reid, W.D.; MacGowan, N.A. Respiratory muscle injury in animal models and humans. Mol. Cell. Biochem. 1998, 179, 63–80.
- Constantin, D.; Menon, M.K.; Houchen-Wolloff, L.; Morgan, M.D.; Singh, S.J.; Greenhaff, P.; Steiner, M.C. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax 2013, 68, 625–633.
- Jagoe, R.T.; Goldberg, A.L. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 183–190.
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708.
- Rabuel, C.; Renaud, E.; Brealey, D.; Ratajczak, P.; Damy, T.; Alves, A.; Habib, A.; Singer, M.; Payen, D.; Mebazaa, A. Human septic myopathy: Induction of cyclooxygenase, heme oxygenase and activation of the ubiquitin proteolytic pathway. Anesthesiology 2004, 101, 583–590.
- Constantin, D.; McCullough, J.; Mahajan, R.P.; Greenhaff, P.L. Novel events in the molecular regulation of muscle mass in critically ill patients. J. Physiol. 2011, 589, 3883–3895.
- Klaude, M.; Mori, M.; Tjader, I.; Gustafsson, T.; Wernerman, J.; Rooyackers, O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin. Sci. 2012, 122, 133–142.
- McKeran, R.O.; Halliday, D.; Purkiss, P.; Royston, P. 3-Methylhistidine excretion as an index of myofibrillar protein catabolism in neuromuscular disease. J. Neurol. Neurosurg. Psychiatry 1979, 42, 536–541.
- Jespersen, J.G.; Nedergaard, A.; Reitelseder, S.; Mikkelsen, U.R.; Dideriksen, K.J.; Agergaard, J.; Kreiner, F.; Pott, F.C.; Schjerling, P.; Kjaer, M. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLoS ONE 2011, 6, e18090.
- Winkelman, C. Inactivity and inflammation in the critically ill patient. Crit. Care Clin. 2007, 23, 21–34.
- Baracos, V.E.; DeVivo, C.; Hoyle, D.H.; Goldberg, A.L. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am. J. Physiol. 1995, 268 Pt 1, E996–E1006.
- Guarnieri, G.; Toigo, G.; Situlin, R.; Del Bianco, M.A.; Crapesi, L. Cathepsin B and D activity in human skeletal muscle in disease states. Adv. Exp. Med. Biol. 1988, 240, 243–256.
- Wagenmakers, A.J. Muscle function in critically ill patients. Clin. Nutr. 2001, 20, 451–454.
- Costelli, P.; Reffo, P.; Penna, F.; Autelli, R.; Bonelli, G.; Baccino, F.M. Ca(2+)-dependent proteolysis in muscle wasting. Int. J. Biochem. Cell. Biol. 2005, 37, 2134–2146.
- Lee, S.J. Regulation of muscle mass by myostatin. Annu. Rev. Cell. Dev. Biol. 2004, 20, 61–86.
- Morissette, M.R.; Cook, S.A.; Buranasombati, C.; Rosenberg, M.A.; Rosenzweig, A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol. Cell Physiol. 2009, 297, C1124–C1132.
- McFarlane, C.; Plummer, E.; Thomas, M.; Hennebry, A.; Ashby, M.; Ling, N.; Smith, H.; Sharma, M.; Kambadur, R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J. Cell Physiol. 2006, 209, 501–514.
- Siu, P.M.; Alway, S.E. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J. Physiol. 2005, 565 Pt 1, 309–323.
- Schwartz, L.M. Skeletal Muscles Do Not Undergo Apoptosis During Either Atrophy or Programmed Cell Death-Revisiting the Myonuclear Domain Hypothesis. Front. Physiol. 2018, 9, 1887.
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412.
- Furuyama, T.; Kitayama, K.; Yamashita, H.; Mori, N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem. J. 2003, 375 Pt 2, 365–371.
- Constantin-Teodosiu, D. Regulation of muscle pyruvate dehydrogenase complex in insulin resistance: Effects of exercise and dichloroacetate. Diabetes. Metab. J. 2013, 37, 301–314.
- Investigators, N.-S.S.; Finfer, S.; Liu, B.; Chittock, D.R.; Norton, R.; Myburgh, J.A.; McArthur, C.; Mitchell, I.; Foster, D.; Dhingra, V.; et al. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 2012, 367, 1108–1118.
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. (1985) 2007, 102, 2389–2397.
- Powers, S.K.; Smuder, A.J.; Criswell, D.S. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid. Redox Signal 2011, 15, 2519–2528.
- Abrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxid. Med. Cell Longev. 2018, 2018, 2063179.
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38.
- Brocca, L.; Pellegrino, M.A.; Desaphy, J.F.; Pierno, S.; Camerino, D.C.; Bottinelli, R. Is oxidative stress a cause or consequence of disuse muscle atrophy in mice? A proteomic approach in hindlimb-unloaded mice. Exp. Physiol. 2010, 95, 331–350.
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559.
- Brooks, S.V.; Faulkner, J.A. Skeletal muscle weakness in old age: Underlying mechanisms. Med. Sci. Sports Exerc. 1994, 26, 432–439.
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open. Biol. 2020, 10, 200048.
- Verdijk, L.B.; Koopman, R.; Schaart, G.; Meijer, K.; Savelberg, H.H.; van Loon, L.J. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E151–E157.
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228.
- Lexell, J.; Taylor, C.C. Variability in muscle fibre areas in whole human quadriceps muscle: Effects of increasing age. J. Anat. 1991, 174, 239–249.
- Frontera, W.R.; Suh, D.; Krivickas, L.S.; Hughes, V.A.; Goldstein, R.; Roubenoff, R. Skeletal muscle fiber quality in older men and women. Am. J. Physiol. Cell Physiol. 2000, 279, C611–C618.
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064.
- Mawson, A.R.; Croft, A.M. Gulf War Illness: Unifying Hypothesis for a Continuing Health Problem. Int. J. Environ. Res. Public Health 2019, 16, 111.
- Ramirez-Sanchez, I.; Navarrete-Yanez, V.; Garate-Carrillo, A.; Loredo, M.; Lira-Romero, E.; Estrada-Mena, J.; Campeau, A.; Gonzalez, D.; Carrillo-Terrazas, M.; Moreno-Ulloa, A.; et al. Development of muscle atrophy and loss of function in a Gulf-War illness model: Underlying mechanisms. Sci. Rep. 2020, 10, 14526.
- Thomson, R.M.; Parry, G.J. Neuropathies associated with excessive exposure to lead. Muscle Nerve 2006, 33, 732–741.
- Nyirenda, M.H.; Morandi, E.; Vinkemeier, U.; Constantin-Teodosiu, D.; Drinkwater, S.; Mee, M.; King, L.; Podda, G.; Zhang, G.X.; Ghaemmaghami, A.; et al. TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: A novel mechanism of reduced regulatory T cell function in multiple sclerosis. J. Immunol. 2015, 194, 5761–5774.
- Mendelson, M.; Nel, J.; Blumberg, L.; Madhi, S.A.; Dryden, M.; Stevens, W.; Venter, F.W.D. Long-COVID: An evolving problem with an extensive impact. S. Afr. Med. J. 2020, 111, 10–12.
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E363–E371.
