Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Aducanumab is a human monoclonal antibody that works to reduce Aβ load in the brain; it is the first disease-modifying therapy to be approved for AD treatment. On 7 June 2021, the United States Food and Drug Administration (U.S. FDA) approved aducanumab via an accelerated approval pathway. Current AD treatment is centered on supportive care to manage the debilitating symptoms of dementia, and pharmacotherapy goals of mainstay classes of drugs, such as cholinesterase inhibitors (ChEIs) and N-methyl-D-aspartate (NMDA) receptor antagonists, do not modify the course of the disease.
- Alzheimer’s disease
- aducanumab
- LMICs
- APOE
- burden of disease
- treatment cost
1. Aducanumab in Low- and Middle-Income Countries
AD places burdens on an individual level, affecting patients, caregivers, and families while also burdening and straining healthcare systems at a societal level. In terms of years of life lived with disability (YLD) due to non-communicable disease, dementia (including AD) accounts for 11.9% of the total number of years [7]. In 2019, AD and all dementias accounted for 5.6% of all global disability-adjusted life years (DALYs) and was the fourth-highest cause of DALYs in patients aged 75 and older [27]. There is a demand for disease-modifying AD therapies, as an increase in the number of dementia and AD cases, especially in LMICs, would exert an unwarranted burden on patients and caregivers. At the same time, resource limitations would negatively impact healthcare systems in these countries. There are currently no biosimilars or generic equivalents of aducanumab. It is the only disease-modifying AD therapy currently in the market, targeting the underlying pathophysiology of the AD disease process [28,29,30]. The economic cost of dementia (including AD) increased by 35.4% from U.S. $604 billion in 2010 to U.S. $818 billion in 2015, which is 1.09% of the world’s GDP [12,31]. An estimated U.S. $715.1 billion or 86% of these costs were from high-income countries (HICs), while LMICs accounted for a sum of U.S. $102.8 billion. A disease-modifying therapy could potentially decrease the burden of AD in terms of mortality and morbidity and improve health outcomes by generating an enduring clinical effect.
Comparing the efficacy of aducanumab directly with more conventional drug classes, such as ChEIs and NMDA receptor antagonists, is challenging, as the pharmacotherapy goals are inherently different. Aducanumab is a recombinant monoclonal antibody based on the principles of passive immunotherapy. It works by selectively binding Aβ fibrils and soluble oligomers, reducing amyloid-beta dose- and time-dependently [32]. ChEIs and NMDA receptor antagonists, on the other hand, aim for symptomatic alleviation. Evidence shows that donepezil, rivastigmine, and galantamine yields modest improvements in cognitive and clinical function in patients with mild to moderate AD in the short and long term [33]. Table 1 represents a crude comparison of the various characteristics of aducanumab, ChEIs, and NMDA receptor antagonists, including pharmacotherapy goals, mechanism of action, and efficacy, among others. The efficacy data are based on the most common primary outcome measures of cognitive function—with aducanumab’s data based on the randomised-control trial (EMERGE), while meta-analyses were used for the remaining drug classes. Care should be taken when interpreting and comparing efficacy between the drugs, as it is subject to variabilities in study design, including patient characteristics and indications. This is due to a lack of head-to-head clinical trials between aducanumab and other drugs currently available on the market; hence, the data in Table 1 that directly compares the efficacy of different drug classes serves an exploratory purpose. Commenting on the robustness of the data presented, the quality of evidence was moderate in studies used in the meta-analyses for donepezil [34] and rivastigmine [35]. There was considerable heterogeneity in some outcome measures in the galantamine [36] and memantine [37] meta-analyses. All meta-analyses presented were subject to publication bias, and most studies included in the analyses were industry-funded.
Table 1. Direct comparison between aducanumab and mainstay pharmacotherapy of AD.
1.1. Accelerated Approval and the Efficacy of Aducanumab
The first aducanumab trials started in 2011 with a phase I study (study 101) after pre-clinical trials with transgenic mice showed reduced amyloid burden in the brain [25,39]. The clinical trials that are important in assessing the efficacy of aducanumab are studies 103 (phase Ib) and the two identical phase III trials: 301 (ENGAGE) and 302 (EMERGE) [40]. Figure 2 is a timeline that summarizes the key events leading up to the accelerated approval of aducanumab.
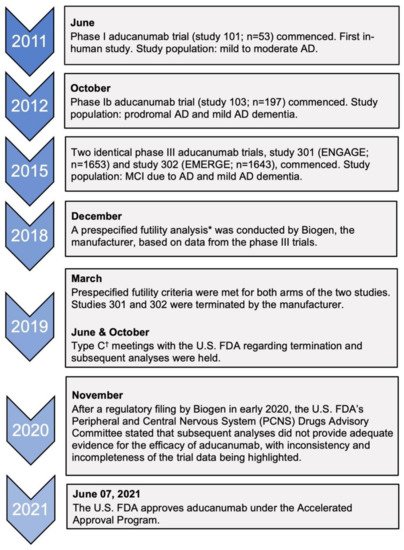
Figure 2. Timeline of clinical trials of aducanumab and key regulatory decisions. AD, Alzheimer’s disease; MCI, mild cognitive impairment. * This was defined as the conditional power being less than 20% for both dose arms of the trials to meet the primary endpoint [40]. † Type C meetings are those that do not fall into type A meetings (due to stalled product development) or type B meetings (for example, after a trial’s end-of-phase) [42].
Of the two phase III clinical trials, only the high-dose arm of one trial, EMERGE, met its primary endpoint by demonstrating improvements in the Clinical Dementia-Sum of Boxes (CDR-SB) score in addition to showing benefits in other secondary outcomes, such as the MMSE score, ADAS-Cog-13, and ADCS-ADL-MCI scores [40]. However, the low-dose arm did not reveal any benefit of aducanumab compared to the placebo, and no benefits were observed in either arm of the ENGAGE trial [29]. In fact, in the ENGAGE trial, it was noted that the CDR-SB score change in the high-dose arm was quantitatively worse than placebo at 78 weeks [40]. Prior to the current analysis by the manufacturer, the two trials were halted in March 2019 after a planned interim analysis met the criteria for futility [41]. On that account, confidence in the efficacy of aducanumab would need to be tempered due to the contradicting evidence presented from the two trials.
Furthermore, the efficacy of aducanumab was determined in research settings, and therefore, clinical practice may vary. The placebo-controlled EMERGE and ENGAGE trial’s population included individuals with early AD (i.e., those with mild cognitive impairment due to AD or those with mild AD) [29]. Moderate to severe AD patients make up approximately 50% of the total number of individuals living with AD [43]. The suitability and efficacy of aducanumab were not assessed in these patient groups, effectively limiting access to half of all patients living with AD, which will continue to contribute significantly to the burden of disease. Additionally, there is an intense debate surrounding the clinical significance observed in the EMERGE trial, as the hypothesis that clearance of Aβ protein equates to clinical improvement is inconclusive and is yet to be demonstrated [14]. As the trials have utilized a surrogate endpoint that facilitated the U.S. FDA’s accelerated approval, the data can only predict a clinical outcome for the treatment of AD. As a result, full approval depends on a phase IV confirmatory trial, which aims to measure the clinical benefit [44]. Consequently, aducanumab’s applicability to a limited subset of AD patients and its currently contended effectiveness would negatively affect the suitability of this drug in many countries, including in LMICs.
1.2. Treatment Challenges
An issue that would impact the suitability of aducanumab in many LMICs is the complexity of the treatment regimen and the need for robust healthcare systems to deliver therapies to patients. Aducanumab is administered by intravenous infusion every four weeks, with increasing titrations every two weeks for the first 32 weeks, followed by a constant high dose beyond week 36, with each infusion lasting 1 h [40]. Additionally, ascertaining the amyloid burden in patients before initiating treatment is crucial to guide clinical diagnosis and to assess the suitability of aducanumab. This is achieved by positron emission tomography (PET) for a visual read or through an invasive cerebrospinal fluid (CSF) quantitative analysis [10]. Amyloid-PET scans should be interpreted cautiously by trained radiologists and nuclear medicine specialists [10]. In addition to resource limitations and healthcare availability and accessibility, this complex treatment regimen highlights the importance of continuity and coordination in healthcare, which is associated with improved health outcomes [45]; however, such measures are lacking in many LMICs [46]. Safety and competency among healthcare workers are essential in delivering novel therapies effectively; evidence suggests that healthcare service competence and safety are deficient in LMICs [45]. The number of physicians per 1000 people in high-income countries was 3.1, while it was 1.3 in low- and middle-income countries [47], further emphasising resource limitations. On the contrary, a prospective advantage of aducanumab’s approval is that it could lay the foundation for future therapies for AD in terms of advancing and improving treatment delivery to patients, which can improve health outcomes. For instance, long-term data from follow-up trials would be crucial in determining efficacy as well as providing an opportunity for head-to-head trials with existing therapies to be conducted. Due to the nature of AD and its multifaceted pathophysiology, combination therapy involving multiple targets may be necessary [48]. Therefore, aducanumab could serve as a catalyst towards better AD treatment in the future. Nonetheless, considering the importance of care continuity, follow-ups, and the general complexities associated with dosing intervals, which are all augmented by a general absence of high-quality healthcare coverage, introducing aducanumab to LMICs would be challenging.
1.3. Adverse Effects
Adequate follow-ups during the treatment phase are crucial for monitoring severe adverse events. ARIA (amyloid-related imaging abnormalities) due to oedema (ARIA-E) and brain microhemorrhage or localised superficial siderosis (ARIA-H) were frequently seen in the treatment groups [40]. In the high-dose treatment arm of the EMERGE and ENGAGE phase III trials, 41.3% of individuals experienced ARIAs compared to 10.3% in the placebo arm [40]. In addition to a pre-treatment MRI, frequent scans were performed to monitor the ARIAs of aducanumab. Individuals were scheduled to have five MRI scans of the brain in the first year of treatment alone, followed by two more scans in the last six months of treatment [40]. In clinical practice, this would mean a pre-treatment MRI, followed by two more brain MRI scans before the seventh and twelfth doses, which is in addition to more scans if patients experience symptoms related to ARIAs. To put it into perspective, in high-income countries, the number of MRI scanners per million inhabitants is 27.3, which contrasts with 3.4 scanners and 0.4 scanners per million inhabitants in upper-middle-income and lower-middle-income countries, respectively, and 0.2 scanners per million inhabitants in low-income countries [49]. If such monitoring practices cannot be implemented effectively in these countries, patients with severe ARIAs would not be identified and managed early, compounding the burden on patients, caregivers, healthcare systems, and economies.
1.4. Apolipoprotein E and Interethnic Differences
The synthesis, clearance, and accumulation of Aβ are influenced by a variety of factors. In the less common familial AD, mutations in the APP gene or PSEN1 gene may lead to increased Aβ accumulation [50]. Age-related processes, including neuronal stress, microglia-related inflammation, and a negative impact on protein homeostasis, may affect Aβ aggregation [51,52,53]. Other factors, such as insulin-like growth factor (IGF) resistance and diabetes, traumatic brain injuries, and the human microbiota, have also been studied [50]. However, for sporadic AD, which is more common, the presence of the ε4 allele of apolipoprotein E (APOEε4) is implicated, while BIN1 (bridging integrator-1) and TREM2 (Triggering Receptor Expressed On Myeloid Cells 2) also play a role [50]. Apolipoproteins usually aid in the transport of lipids in the body; however, APOE4 can also form stable complexes with Aβ, impacting its clearance from the brain. Therefore, APOEε4 genotype carriers have a higher amyloid load than non-carriers, and amyloid positivity is associated with greater cognitive impairment [54,55].
In the EMERGE and ENGAGE trials, participants underwent genetic screening for the presence of the APOEε4 allele [30]. It was noted that 65–75% of patients with AD carry the APOEε4 allele [56]. For instance, Mattsson and colleagues’ [57] meta-analysis found that the frequency of APOEε4 carriers in patients with AD was 68.9% in Northern Europe and 52.1% in Asian populations. For patients with MCI, this was 52.5% vs. 33.3% in Northern Europe vs. Asia. The proportion of APOEε4 carriers with MCI who were Aβ positive in both Asian and European populations were significant compared to patients who were Aβ negative (Figure 3a,b) [57]. This is important, as the population assessed in the aducanumab trials were patients with confirmed amyloid pathology [10], which relates to the mechanism of action of the therapeutic. While more studies need to be conducted to confirm differences in APOEε4 allele frequencies across different populations, it may indicate the relative prevalence of amyloid pathology across different regions and ethnicities and, by extension, may guide cost-effectiveness assessments in nations including in LMICs.
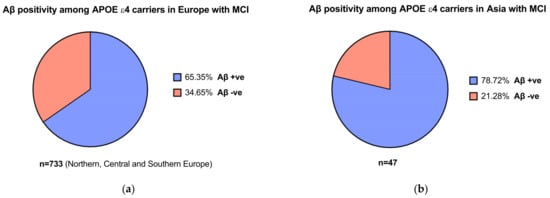
Figure 3. (a) Aβ positivity among APOEε4 carriers with mild cognitive impairment (MCI) in Europe (b) and in Asia. Data obtained from Mattson et al. [57].
Most notably, APOEε4 status was associated with an increased incidence of ARIA-E. As a result, dosing was adjusted in the initial stages of the trials to allow for lower doses in participants who carried the gene [29]. Figure 4 represents the number of participants who experienced ARIA-E in EMERGE and ENGAGE, stratified by APOEε4 carrier status compared to placebo. Furthermore, homozygous carriers may be predisposed to more frequent and severe ARIA than those who carry only one copy of the allele [58]. Although genotype testing is currently not indicated as part of the pre-treatment procedure, clinicians must carefully assess the risk-benefit balance. Additionally, if such risk-stratification measures are required as part of a full regulatory approval in the future, it would only serve to compound resource and economic burdens when delivering this complex therapy to patients.
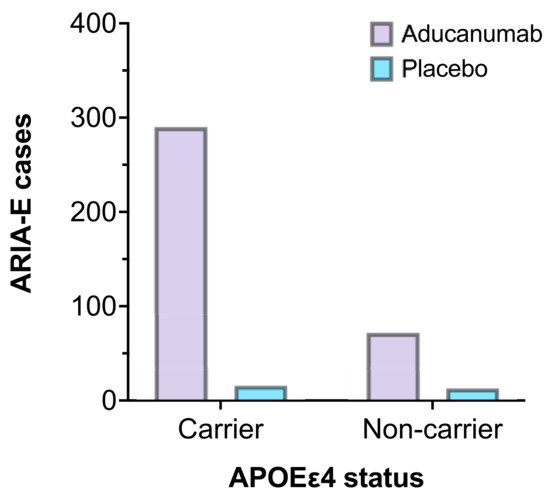
Figure 4. The number of ARIA-E cases by APOEε4 carrier status in EMERGE and ENGAGE (aducanumab 10 mg/kg, n = 362 vs. placebo, n = 29) [44].
The ethno-racial differences in AD pathology could impact the cost-effectiveness of aducanumab in LMICs. In diagnostics, P-tau biomarkers (p-tau181 and p-tau217) were associated with Aβ pathology on PET [59]. Brickman et al. [59] found that concentrations of p-tau biomarkers did not differ across Non-Hispanic Whites, Black people, and Hispanics. However, another study found that Black people had lower CSF phosphorylated-tau (p-tau181) and total tau (t-tau) levels when compared to Caucasians, independent of cognition [60]. Although there is no clear association between the prevalence of amyloid pathology and socioeconomic status, and due to limitations in the literature concerning the interethnic impact of both allele frequencies and biomarker levels on amyloid pathology, aducanumab’s use in these populations must be assessed to further clarify its cost-effectiveness, especially in the context of LMICs.
1.5. The Economic Burden
As announced by the manufacturer, aducanumab’s wholesale cost is U.S. $56,000 annually per patient [30]. The Institute for Clinical and Economic Review’s (ICER) value-based analysis suggests a significantly lower price between U.S. $2500 and U.S. $8300 per annum, which is comparable to existing classes of drugs [30] (Table 2). The prevalence of AD is significant; however, its relatively low incidence compared to other non-communicable diseases could mean lower total public health expenditure. Nevertheless, ICER’s report estimated the annual United States budget impact to be U.S. $819 million for aducanumab [30]. There is a pronounced incongruity between pharmaceutical spending in high-income countries and LMICs—while 84% of the global population reside in LMICs, it only accounted for 21.5% of the total global pharmaceutical expenditure [61]. Since aducanumab targets the early AD stage to prolong disease progression [40], healthcare systems would need to factor in the costs of managing patients over a longer period. Training individuals regarding safe administration, monitoring, follow-ups, and best practices would incur high costs. Moreover, the costs associated with pre-and post-treatment follow-ups, such as the need for multiple MRI scans of the brain, as described, are likely to burden national healthcare systems and economies while serving as a barrier by making healthcare inaccessible to patients. Conversely, the epidemiological shifts seen in LMICs towards lower mortality rates [13] may reflect improving economies, which could indicate a better level of economic sustainability for drugs such as aducanumab. Nevertheless, determining and comparing the cost-effectiveness of aducanumab between countries is challenging due to the different architectures of healthcare systems, inconsistent funding, utilisation of resources, and the individual nature of policy and legislation development.
Table 2. Additional differences between aducanumab and conventional drugs for the treatment of AD.
This entry is adapted from the peer-reviewed paper 10.3390/brainsci11111547
This entry is offline, you can click here to edit this entry!
