The Powassan virus (POWV) is a rare tick-borne virus that can cause severe neurological damage and death, and the incidence of the associated disease (Powassan virus disease) is increasing in the eastern United States. The mechanisms by which POWV is maintained in nature and transmitted to humans are complex and only partly understood.
- powassan virus
- deer tick virus
- ticks
- tick-borne virus
1. Introduction
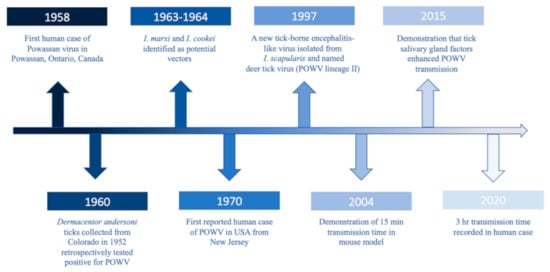
POWV is a neuroinvasive, single-stranded, positive sense RNA tick-borne flavivirus (Flaviviridae), and it is the only member of the tick-borne encephalitis serogroup in North America. POWV was first isolated in 1958 from the brain of a five-year-old boy from Powassan, Ontario who died of encephalitis (Figure 1) [1]. In 1970, the first human case of POWV in the US was reported in New Jersey (Figure 1) [2]. Approximately two decades later, a tick-borne encephalitis-like virus was detected in Ixodes scapularis (deer ticks) which was genetically different than POWV and thus was named deer tick virus (Figure 1) [3]. Subsequently, it was discovered that POWV had two lineages: Powassan virus lineage I (POWV-LB), isolated from the first case, and Powassan virus lineage II (DTV) isolated from I. scapularis ticks [3]. While the two lineages share 84% nucleotide identity and 94% amino acid sequence [4], they are serologically indistinguishable [5] and are both diagnosed as Powassan virus.
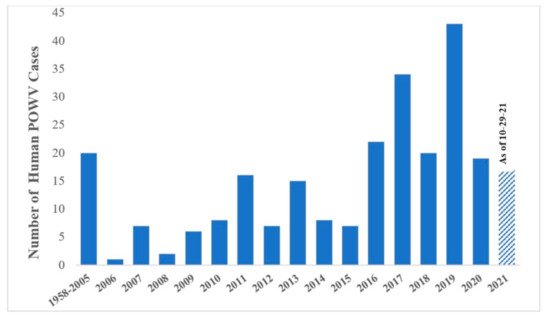
Clinical cases of POWV are defined by the CDC as having a fever, central and peripheral nervous system dysfunction, and the absence of a more likely clinical explanation [6]. Human cases are restricted to the distribution range of the tick vector, namely in the Northeast and Upper Midwest states, and they are rising in incidence (Figure 2). Prior to 2006, only 20 cases were reported to the CDC [7]. However, between 2010 and 2019 alone, 181 cases were reported [8]. This increase is likely due to increased surveillance and reporting (POWV neuroinvasive and non-neuroinvasive diseases were added to the list of nationally notifiable diseases in 2001 and 2004, respectively), improved diagnosis, and/or increased prevalence [7][9]. With POWV disease being a rare tick-borne disease that can also have non-specific symptoms, it is likely that cases are underestimated and/or misdiagnosed, and the true extent of the case geographic distribution cannot be determined [10].
Unlike B. burgdorferi, POWV can be deadly, with a 3–35.7% case fatality rate and long-term neurological damage in 50% of survivors [10][12]. Importantly, both strains of the virus can cause fatal neurological disease [12][13], and common symptoms can include encephalitis, meningitis, aseptic meningitis, febrile illness, lethargy, weakness, confusion, headaches, and vomiting [7][14].
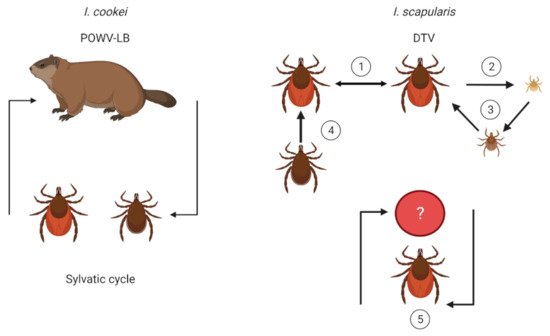
2. Vector and Host Associations
3. Transmission Dynamics
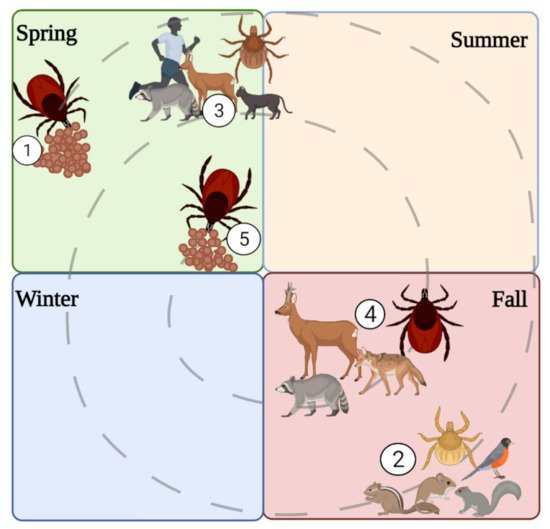
4. Spatial, Temporal, Habitat, and Meteorological Associations
5. Strain Variation and Stability
6. Climate Change and Anthropogenic Influence
In the US, ecological and climate modeling has predicted a growing distribution of I. cookei northward into Canada and a decreasing presence southward in the US, while I. scapularis is predicted to expand northward and westward [36], exposing new areas to tick-borne pathogens. Similarly, the white-footed mouse is expected to also expand northward and colonize new areas as temperature rises and winter length shortens [43], and this northward movement may be supported by earlier oak flowering which is correlated to rising spring temperatures earlier in the year [44]. It is possible that this effect may support other rodent species and pathogen reservoirs. Also, with increasing temperatures and shorter winters, groundhogs may exhibit less time in hibernation and more time actively outside of burrows, reproducing [45]. However, whether this has an impact on I. cookei population density or POWV prevalence is unknown.
With decreasing forest patch sizes, we may expect to see increased densities of I. scapularis [46], though fragmentation effects on POWV are unknown since the virus presents focally. Moreover, its effect on I. cookei is undetermined since the tick may effectively be sustained in burrows. Furthermore, without knowing the reservoir for DTV, we cannot be sure how habitat loss directly impacts DTV prevalence.
While humans are contributing to global climate change and habitat reduction, increasing tick-borne disease prevalence, there are a number of ways that people can physically reduce tick densities and personal tick-borne pathogen acquisition, including POWV. Personal preventative measures for reducing pathogen attainment include frequently checking for ticks when outdoors, staying in the center of maintained trails, wearing EPA-approved repellents, wearing light-colored clothing, and tucking one’s pants into their socks [47]. Prevention methods at home include cultivating a tick-free yard space by reducing leaf litter on the ground, removing weeds and bramble that may attract small rodents, creating a mulch barrier between the woods and the yard, and allowing ample sunlight to dry out the yard (reducing moisture for ticks) by regularly mowing and landscaping properly [47]. Host-targeted acaricides also are available to treat rodents and deer through passive topical applications [47]. By reducing hosts, treating hosts for ticks, landscaping to reduce tick abundance, and practicing personal prevention methods, tick-borne pathogen spillover to humans can be reduced. However, these methods are on the individual level, and long-term solutions require global participation.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9112317
References
- McLean, D.M.; Donohue, W.L. Powassan virus: Isolation of virus from a fatal case of encephalitis. Can. Med. Assoc. J. 1959, 80, 708–711.
- Goldfield, M.; Austin, S.M.; Black, H.C.; Taylor, B.F.; Altman, R. A non-fatal human case of Powassan virus encephalitis. Am. J. Trop. Med. Hyg. 1973, 22, 78–81.
- Telford, S.R.; Armstrong, P.M.; Katavolos, P.; Foppa, I.; Garcia, A.S.O.; Wilson, M.L.; Spielman, A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg. Infect. Dis. 1997, 10, 156–157.
- Kuno, G.; Artsob, H.; Karabatsos, N.; Tsuchiya, K.R.; Chang, G.J.J. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am. J. Trop. Med. Hyg. 2001, 65, 671–676.
- Beasley, D.W.C.; Suderman, M.T.; Holbrook, M.R.; Barrett, A.D.T. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res. 2001, 79, 81–89.
- CDC. Arboviral Diseases, Neuroinvasive and Non-Neuroinvasive 2015 Case Definition. 2021. Available online: https://ndc.services.cdc.gov/case-definitions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive-2015/ (accessed on 3 November 2021).
- Krow-Lucal, E.R.; Lindsey, N.P.; Fischer, M.; Hills, S.L. Powassan virus disease in the United States, 2006–2016. Vector-Borne Zoonotic Dis. 2018, 18, 286–290.
- CDC. Powassan Virus. Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/powassan/statistics.html (accessed on 7 June 2021).
- Hinten, S.R.; Beckett, G.A.; Gensheimer, K.F.; Pritchard, E.; Courtney, T.M.; Sears, S.D.; Woytowicz, J.M.; Preston, D.G.; Smith, R.P.; Rand, P.W.; et al. Increased recognition of powassan encephalitis in the United States, 1999–2005. Vector-Borne Zoonotic Dis. 2008, 8, 733–740.
- Corrin, T.; Greig, J.; Harding, S.; Young, I.; Mascarenhas, M.; Waddell, L.A. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health 2018, 65, 595–624.
- CDC. Powassan Virus. 2021. Available online: https://wwwn.cdc.gov/arbonet/Maps/ADB_Diseases_Map/index.html (accessed on 7 June 2021).
- Ebel, G.D. Update on Powassan virus: Emergence of a North American tick-borne flavivirus. Annu. Rev. Entomol. 2010, 55, 95–110.
- Hermance, M.E.; Thangamani, S. Powassan virus: An emerging arbovirus of public health concern in North America. Vector-Borne Zoonotic Dis. 2017, 17, 453–462.
- El Khoury, M.Y.; Camargo, J.F.; White, J.L.; Backenson, B.P.; Dupuis, A.P.; Escuyer, K.L.; Kramer, L.; George, K.S.; Chatterjee, D.; Prusinski, M.; et al. Potential Role of Deer Tick Virus in Powassan Encephalitis Cases in Lyme Disease–endemic Areas of New York, USA. Emerg. Infect. Dis. 2013, 19, 1926–1933.
- Mclean, D.M.; Walker, S.J.; Macpherson, L.W.; Scholten, T.H.; Ronald, K.; Wyllie, J.C.; Mcqueen, E.J. Powassan Virus: Investigations of possible natural cycles of infection. J. Infect. Dis. 1961, 109, 19–23.
- McLean, D.M.; Larke, R.P. Powassan and Silverwater viruses: Ecology of two Ontario arboviruses. Can. Med. Assoc. J. 1963, 88, 182–185.
- Main, A.J.; Carey, A.B.; Downs, W.G.; Haven, N. Powassan virus in Ixodes cookei and mustelidae in New England. J. Wildl. Dis. 1979, 15.
- Mclean, D.M.; de Vos, A.; Quantz, J. Powassan virus: Field investigations of 1963. Am. J. Trop. Med. Hyg. 1964, 13, 747–753.
- Johnson, H.N. Isolation of Powassan virus from a spotted skunk in California. J. Wildl. Dis. 1987, 23, 152–153.
- Dupuis, A.P.; Peters, R.J.; Prusinski, M.A.; Falco, R.C.; Ostfeld, R.S.; Kramer, L.D. Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasites Vectors 2013, 6, 185.
- Ebel, G.D.; Campbell, E.N.; Goethert, H.K.; Spielman, A.; Telford, S.R. Enzootic transmission of deer tick virus in new England and Wisconsin sites. Am. J. Trop. Med. Hyg. 2000, 63, 36–42.
- Deardorff, E.R.; Nofchissey, R.A.; Cook, J.A.; Hope, A.G.; Tsvetkova, A.; Talbot, S.L.; Ebel, G.D. Powassan Virus in mammals, Alaska and New Mexico, USA, and Russia, 2004–2007. Emerg. Infect. Dis. 2013, 19, 1–5.
- Frey, S.; Essbauer, S.; Zöller, G.; Klempa, B.; Dobler, G.; Pfeffer, M. Full genome sequences and preliminary molecular characterization of three tick-borne encephalitis virus strains isolated from ticks and a bank vole in Slovak Republic. Virus Genes 2014, 48, 184–188.
- Weidmann, M.; Schmidt, P.; Hufert, F.T.; Krivanec, K.; Meyer, H. Tick-borne encephalitis virus in Clethrionomys glareolus in the Czech Republic. Vector-Borne Zoonotic Dis. 2006, 6, 379–381.
- Ebel, G.D.; Kramer, L.D. Short report: Duration of tick attachment required for transmission of powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 2004, 71, 268–271.
- Hermance, M.E.; Thangamani, S. Tick−Virus−Host Interactions at the Cutaneous Interface: The Nidus of Flavivirus Transmission. Viruses 2018, 10, 362.
- Feder, H.M.; Telford, S.; Goethert, H.K.; Wormser, G.P. Powassan virus encephalitis following brief attachment of Connecticut deer ticks. Clin. Infect. Dis. 2020.
- Spielman, A.; Ribeiro, J.M.C.; Mather, T.N.; Piesman, J. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 1987, 24, 201–205.
- Hermance, M.E.; Thangamani, S. Tick saliva enhances Powassan virus transmission to the host, influencing its dissemination and the course of disease. J. Virol. 2015, 89, 7852–7860.
- Costero, A.; Grayson, M.A. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari:Ixodidae). Am. J. Trop. Med. Hyg. 1996, 55, 536–546.
- Woodall, J.P.; Roz, A. Experimental milk-borne transmission of Powassan virus in the goat. Am. J. Trop. Med. Hyg. 1977, 26, 190–192.
- Dorko, E.; Hockicko, J.; Rimárová, K.; Bušová, A.; Popaďák, P.; Popaďáková, J.; Schréter, I. Milk outbreaks of tick-borne encephalitis in Slovakia, 2012-2016. Cent. Eur. J. Public Health 2018, 26, S47–S50.
- Cisak, E.; Wójcik-Fatla, A.; Zając, V.; Sroka, J.; Buczek, A.; Dutkiewicz, J. Prevalence of tick-borne encephalitis virus (TBEV) in samples of raw milk taken randomly from cows, goats and sheep in eastern Poland. Ann. Agric. Environ. Med. 2010, 17, 283–286.
- Pak, D.; Jacobs, S.B.; Sakamoto, J.M. A 117-year retrospective analysis of Pennsylvania tick community dynamics. Parasites Vectors 2019, 12, 1–14.
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386.
- Alkishe, A.; Raghavan, R.K.; Peterson, A.T. Likely geographic distributional shifts among medically important tick species and tick-associated diseases under climate change in North America: A review. Insects 2021, 12, 225.
- Simmons, T.W.; Shea, J.; Myers-Claypole, M.A.; Kruise, R.; Hutchinson, M.L. Seasonal activity, density, and collection efficiency of the blacklegged tick (Ixodes scapularis) (Acari: Ixodidae) in Mid-Western Pennsylvania. J. Med. Entomol. 2015, 52, 1260–1269.
- CDC. How Ticks Spread Disease. 2020. Available online: https://www.cdc.gov/ticks/life_cycle_and_hosts.html (accessed on 10 October 2021).
- Pesko, K.N.; Torres-Perez, F.; Hjelle, B.L.; Ebel, G.D. Molecular epidemiology of Powassan virus in North America. J. Gen. Virol. 2010, 91, 2698–2705.
- Anderson, J.F.; Armstrong, P.M. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am. J. Trop. Med. Hyg. 2012, 87, 754–759.
- Borde, J.P.; Kaier, K.; Hehn, P.; Matzarakis, A.; Frey, S.; Bestehorn, M.; Dobler, G.; Chitimia-Dobler, L. The complex interplay of climate, TBEV vector dynamics and TBEV infection rates in ticks—Monitoring a natural TBEV focus in Germany, 2009–2018. PLoS ONE 2021, 16, e0244668.
- Brackney, D.E.; Nofchissey, R.A.; Fitzpatrick, K.A.; Brown, I.K.; Ebel, G.D. Short report: Stable prevalence of Powassan virus in Ixodes scapularis in a Northern Wisconsin focus. Am. J. Trop. Med. Hyg. 2008, 79, 971–973.
- Roy-Dufresne, E.; Logan, T.; Simon, J.A.; Chmura, G.L.; Millien, V. Poleward expansion of the white-footed mouse (Peromyscus leucopus) under climate change: Implications for the spread of lyme disease. PLoS ONE 2013, 8.
- Caignard, T.; Kremer, A.; Firmat, C.; Nicolas, M.; Venner, S.; Delzon, S. Increasing spring temperatures favor oak seed production in temperate areas. Sci. Rep. 2017, 7, 1–8.
- Zervanos, S.M.; Maher, C.R.; Waldvogel, J.A.; Florant, G.L. Latitudinal differences in the hibernation characteristics of woodchucks (Marmota monax). Physiol. Biochem. Zool. 2010, 83, 135–141.
- Allan, B.F.; Keesing, F.; Ostfeld, R.S. Effect of Forest Fragmentation on Lyme Disease Risk. Conserv. Biol. 2003, 17, 267–272.
- Stafford, K.C. Tick Management Handbook. The Connecticut Agricultural Experiment Station, 71. 2004. Available online: http://www.ct.gov/caes/lib/caes/documents/special_features/tickhandbook.pdf (accessed on 10 July 2021).
