Microbial fuel cells (MFCs) are electrochemical devices focused on bioenergy generation and organic matter removal carried out by microorganisms under anoxic environments. In these types of systems, the anodic oxidation reaction is catalyzed by anaerobic microorganisms, while the cathodic reduction reaction can be carried out biotically or abiotically. Membranes as separators in MFCs are the primary requirements for optimal electrochemical and microbiological performance.
- membranes
- microbial fuel cell
- proton-exchange membrane
- separators
1. Introduction
Nowadays, fossil fuels are extensively; however, they are not a renewable source of energy. Furthermore, fossil fuels have caused social and environmental problems [1][2]. To mitigate these effects, research efforts supporting renewable energy sources can suggest alternatives [3][4][5], notwithstanding the limitations of natural resources in certain regions of the globe. The feasibility of improving the performance of these renewable energy sources relies on integrating suitable and efficient energy storage systems (zero CO 2 emissions) capable of storing unstable energy generated through options such as lithium-ion batteries [6], iron-based redox flow batteries [7], and super-capacitors [8]. Integration of these systems requires trade-offs between the physical and chemical fundamentals of energy generation and its storage through advanced systems beyond the capacity of fuel cells (FCs).
At the beginning of the 19th century, FCs appeared as a green energy technology. FCs represent a way of producing clean energy, which implies zero CO 2 emissions. These electrochemical systems transform the chemical energy stored in H 2 into electricity (Equation (1)), with water (Equation (2)) and large amounts of heat as by-products (Equation (3)), formed as a result of the oxidation–reduction process [9]. (1) H 2→ 2H + + 2e − Hydrogen oxidation (2) 12O 2+ 2H + + 2e − → H 2O Oxygen reduction (3) H 2+ 12O 2→ H 2O + Δ H ° f = − 285.8 kJ / mol Global oxidation – reduction reaction
Nowadays, Nafion ® 117 (NF-117) membranes have the optimal characteristics required for MFCs [10][11]. However, despite the fact that NF-117 membranes the best available membranes for MFCs, their high price discourages their use once MFCs are scaled-up, limited by increases in R i n t [12][13]. At the time of choosing a membrane for MFC applications, it should meet several selection criteria such as outstanding mechanical and chemical stability, no electronic conduction, impermeability to gases such as H 2 and N 2, partial hydrophilicity, easy acquisition, high ionic conductivity, high species selectivity, low oxygen and fuel crossover, and low cost and electrical resistance [14][15][16][17]. Undesirably, membrane use increases the R i n t of the MFC due to the influence of the thickness of the membrane. Phenomena such as biofouling and fuel crossover also contribute to increases in the R i n t of MFCs during their operation. Sun and Zhang [18] demonstrated the influence of membrane thickness on some physicochemical properties; they used three commercial Nafion ® membranes: Nafion ® 212, Nafion ® 115, and NF-117, with different thicknesses 50, 126, and 178 μm, respectively. They observed that as the membrane thickness increases, the membrane resistance increases, and the proton conductivity decreases; however, a greater thickness is useful for restraining interpenetration of electro-active species. Additionally, they observed that the three membranes evaluated with different thicknesses showed similarly high levels of chemical stability. Therefore, high cost and the influence of membrane thickness on the R i n t of MFCs are two big challenges to overcome. In search of new membranes or separators able to provide/produce a similar performance to Nafion ® and reduce cost, many alternatives have been studied. Some of the membranes and separators assessed in MFCs by researchers are the following: cation-exchange membranes (e.g., sulphonated poly(ether ether ketone membranes SPEEKs )), Selemion HSFs, and polystyrene and divinylbenzene with sulfonic acid groups), anion-exchange membranes (e.g., Zirfon ® ), ultrafiltration and microfiltration membranes, bipolar membranes, forward osmosis membranes, cloth (J-cloth) separators, glass fiber separators, cation-exchange layers made of purified kaolin, porous porcelain coated with Nafion ® 117 solution, dialysis membranes, thin layer spray-coating of hydrophilic cation-exchange polymers, anion-exchange and neutral polymers, porous fabrics and coarse-pore filter material, polytetrafluoroethylene membranes, isopore membrane filters, biomax ultrafiltration discs, glass wool, nylon membranes, polycarbonate membranes, cellulose nitrate membranes, kaolin, porcelain and polyethylene membrane interpolymers, forward osmosis membranes, and agar–agar membranes [1][2][19][10][11][20][21][13][15][22][23][24][25][26][27][28][29][30]. The aim of these studies is to lessen costs, reduce R i n t , increase P output and Coulombic efficiency ( η c o u l ), and to improve the membrane separator as a key component [30].
This work aims to critically review the state of the art on membranes and separators used in MFCs and their implications. The scope of this work includes the review of (i) membrane and separator functions in MFCs, (ii) the most-used membranes, (iii) membrane cost and efficiency, and (iv) membrane-less MFCs.
2. Microbial Fuel Cell Components and Basic Functioning
In general, a membrane acts as a thin physical barrier with a <200 µm thickness; it separates the fluids between the anodic and cathodic chambers, defined as anolyte and catholyte, respectively, where oxidation and reduction reactions take place. Nevertheless, complete separation is not observed.
The membrane should not allow a physical interaction between the respective electrolytes, but rather should only aid in the transfer of ions (anions and/or cations) via electro-osmotic drag between the two MFC sections. Membranes must inhibit mass transfer between chambers; membrane performance depends on their physical and chemical properties. In the case of membranes with pores in their structure, membrane performance is the function of pore size and the number of pores (porosity). Nevertheless, there are nonporous membranes where porosity is conceptualized as the phase-separation degree between hydrophobic and hydrophilic phases, playing a significant role in membrane performance. The size of ion clusters (size of ion transport channel/pathways), and ion-exchange capacity (IEC) are other important factors to consider when evaluating nonporous membrane performance. Porous membranes do not have functional groups; therefore, they do not have IEC [31]. Thus, depending on the presence of pores, membranes have been classified into two groups: porous and nonporous membranes. The nonporous membranes, also called ion-exchange membranes (IEMs), are in turn classified into three groups based on the type of ion that is transferred: cation-exchange membranes (CEMs), anion-exchange membranes (AEMs), and bipolar membranes (BPMs). On the other hand, porous membranes have been grouped into ultrafiltration membranes (UFMs), microfiltration filtration membranes (MFMs), ceramic membranes (CMs), and pore filter materials [14][11][16].
A similar phenomenon related to the substrate used as fuel in the anode chamber should be avoided: “fuel crossover”. In this case, the function of the membrane is to prevent soluble low molecular weight organic compounds from being transferred from the anode to the cathode ( Figure 1 ) [10]. Additionally, another function of the membranes used in MFCs that is poorly discussed is in avoiding the crossing or exchange of microorganisms between chambers. In general, the success of a membrane within an MFC will depend mainly on its transport characteristics, i.e., it will depend on its ability to inhibit the transfer of certain species between chambers (substrate and oxygen) but allow the passage of others (protons or anions).
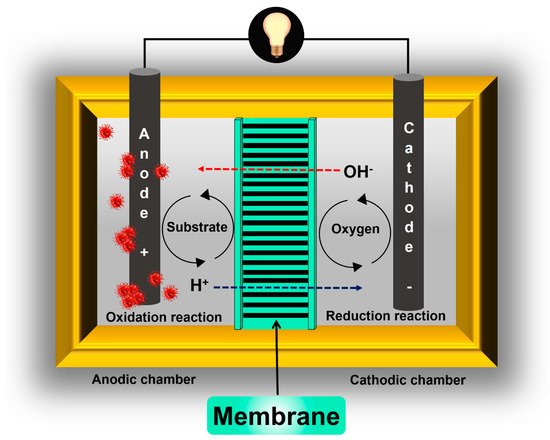
On the other hand, the fuel usually used as the anolyte in MFCs has several organic and sometimes inorganic species. Additionally, microorganisms are essential fuel components—they are the catalyzers that carry out the oxidation process that occurs at the anodic chamber. Therefore, the membrane should have good chemical stability to prevent membrane oxidation and microbiological degradation [32].
3. Membrane-Less Microbial Fuel Cells
In PE MFCs, the separation of H 2 and O 2 gases is essential. This important task is carried out by a membrane that must be able to conduct the protons produced by the oxidation of H 2 (Equation (1)) towards the cathode, for the corresponding simultaneous reduction of O 2 (Equation (2)). A CEM, also referred to as a PEM for its assigned function (proton transfer), is the membrane of choice. However, in the case of MFCs, the membrane could be an unnecessary component of the system configuration. MFCs without a membrane were one of the first configurations used in the principle of MFCs [19][29]. This modification to the system is based on the property of water transfer of H + from the anode chamber to the cathode chamber directly through the system [10][33][34].
As previously analyzed, the use of nonporous membranes or porous membranes generates a series of electrochemical, microbiological, and physicochemical problems within MFCs. The main factor affected by the presence of a membrane or separator is the R i n t . By itself, the membrane has an R value. When placed inside an MFC, this value is added to the other resistances of the system, increasing R i n t [29][35]. However, the value of R is not constant within the system. Its value changes negatively for the system throughout the operation time because of the biofouling formation that indirectly increases the thickness of the membrane; therefore, the value of R i n t increases even more. Besides, biofouling decreases the σ, generating another phenomenon—pH splitting [36][37]. Thus, the use of the membrane and its associated effects causes MFC performance to decrease. Another important parameter that is not related to the MFC’s performance is related to its cost. Depending on the type of membrane chosen for use in an MFC, the construction and operation costs increases considerably by up to 60% if NF-117 is used [38][17]. Due to the negative effects associated with the presence of membranes within the configuration of MFCs and the ability of water to transfer protons, the option of not using membranes and operating the MFCs without the use of them has been considered [1][39]. Furthermore, the elimination of membranes or separators in the design of MFCs responds to the need to reduce the cost of the devices and simplify their design, both of which are critical factors for the scaling and application of these devices in real scenarios [1][40][41][42][43][44].
When membrane-less MFCs are connected in series, the arrangement is inefficient, because it is not possible to equalize the sum of the Δ V produced by the individual cells. Therefore, these devices require design improvements [45]. However, at the start of the operation of a membrane-less MFC, the main effect observed is on the R i n t ; its value decreases considerably and in turn is reflected in a notable increase in the P MFC [46]. Additionally, a lower investment cost in the construction of membrane-less MFCs is attractive [14][16]. Despite the apparent advantages of operating MFCs without membranes, in relatively short operating times, the price of not using a membrane is reflected in a considerably lower value of η c o u l compared to a cell operated with a membrane. Without the membrane, the proton transfer rate from the anode to the cathode is high and two undesirable phenomena appear due to the lack of a barrier between the two half-reactions: fuel crossover and OD. The highest fuel and oxygen crossover values have been observed in this type of configuration. OD and fuel crossover are mainly responsible for the considerable decrease in MFC performance in terms of η c o u l [33][34].
As previously discussed, the presence of oxygen at the anode competes for the electrons generated by oxidation. Besides, the crossover fuel towards the cathode decreases the anodic fuel load available to be anaerobically oxidized by electrochemically active bacteria and harvests the electrons through the external circuit of the system [1][2][39][10][11][47]. The fuel crossover generates a biofilm on the cathode surface, and the interaction of oxygen with the cathode surface decreases, i.e., the biofilm prevents oxygen from acting as an electron acceptor at the cathode [48]. Also, in membrane-less MFCs values of η C O D > 90% have been reported—a significantly high η C O D value. However, this η C O D is attributed to a mainly aerobic oxidation process. Organic matter is oxidized under aerobic conditions at the cathode, therefore, the oxidation rate and η C O D are higher [17]. In the work of Ghangrekar and Shinde [40], the performance of a device without mediators or membranes using synthetic and raw WW , an η C O D ca. 90% was achieved. The high value of η C O D does not justify the operation of MFCs. If this were the case, we would be talking about aerobic oxidation systems that do not need to be operated or be constructed as MFCs, but as simple aerobic oxidation systems where the process is carried out within the same system. In summary, the presence of a membrane greatly reduces the phenomena of fuel and oxygen crossover, improving the η c o u l [39]. The use of a membrane in MFCs is not essential; nevertheless, the researchers concluded that a separator or membrane is necessary to ensure an efficient and sustainable MFC operation [10][49].
4. Conclusions and Outlook
Studies on MFCs as new green eco-friendly technology have awoken huge interest. Although their performances are low compared to the Ps generated by PEMFCs, the scientific community continues to look for improving the MFC performance. The interest in this technology is based on potential WW treatment under anaerobic conditions and the generation of bioelectricity, i.e., the conversion of organic pollutants into energy and the generation of effluent with a lower content of organic matter. Nevertheless, several factors directly impact MFC performance. The membrane or separator used in these devices represents a key part of proper operation and good performance. So far, Nafion ® membranes (perfluorinated membranes), a type of PEM, have been shown to have the best characteristics in terms of performance ( η c o u l and P M F C ) for MFCs. However, an important issue that membranes present during their operation is their high cost, which limits their application. Despite the high cost, the NF-117 membrane is the model membrane in MFCs. The main properties of the Nafion ® membrane are relatively high σ, chemical stability, and excellent mechanical properties. The search to synthesize membranes or separators of low cost and with attractive performances as alternatives to NF-117 is still in progress. Some considerations discussed previously must be taken into account to propose new alternative membranes or separators. The development of new alternative membranes to apply in MFCs is a difficult task. However, finding a new membrane able to reach competitive MFC performance at a low cost is necessary for scaling up. A membrane must perform a lot of characteristics to be considered as an ideal membrane.
A membrane could be considered ideal for use in MFCs if it has the following properties: a high σ, low water and substrate loss, low thickness, impermeability to oxygen and cations such as N H 4 + , N a + , K + , M g 2+ , and C a 2 + , good mechanical properties, chemical stability, low R i n t values, impermeability to gases such as H 2, N 2, and, definitely, a low price. Meeting all these properties simultaneously is difficult. However, this search focuses on finding membranes that have the greatest number of positive attributes and, above all, whose use reflects a good MFC performance in terms of low average R i n t , long stability or durability over the operation time under different operating conditions, biofouling resistance, being nonbiodegradable, low cost, and high η c o u l . Unfortunately, there is no ideal membrane. The IEMs, especially the PEMs, are the membranes that depict some of the most important properties, and this allows the membranes to be applied directly in MFCs. The proposed porous membranes and other separators are not the best choices as ion-exchange separators to be used in MFCs.
The presence of a membrane as a separator in MFCs is not essential. However, it significantly improves MFC performance, although its use has a direct impact on initial investment and maintenance costs. Again, a trade-off solution should be accomplished. Although the use of membranes increases the cost, it improves performance. Finally, despite all the advantages of MFCs (wastewater treatment, bioenergy generation as bioelectricity, C H 4 or H 2, and heavy metals removal), the low Ps and the cost of the membranes still represent a challenge for a suitable scaling up of this technology. The presence of membranes or separators as a part of MFC configurations contributes to the operation of the MFCs for prolonged periods and favors the electrochemical performance. Thus, although the use of a membrane shows several drawbacks as part of the MFC configuration, its use is highly recommended. There are many membranes proposed for use in MFCs. However, the characteristics and performances observed lead us to suggest that the best strategy is to develop membranes focused on the synthesis of IEMs using low-cost materials (organic polymers) with high species selectivity and low R, but high biofouling resistance and good chemical stability.
This entry is adapted from the peer-reviewed paper 10.3390/membranes11100738
References
- Logan, B.E.; Regan, J.M. Microbial challenges and harnessing the metabolic activity of bacteria can provide energy for a variety of applications, once technical and cost obstacles are overcome. Environ. Sci. Technol. 2006, 5172–5180.
- Yang, Y.; Sun, G.; Xu, M. Microbial fuel cells come of age. J. Chem. Technol. Biotechnol. 2010, 86, 625–632.
- Cheng-Dar, Y.; Chung-Ming, L.; Liou, E.M.L. A transition toward a sustainable energy future: Feasibility assessment and development strategies of wind power in Taiwan. Energy Policy 2001, 29, 951–963.
- Tye, Y.Y.; Lee, K.T.; Abdullah, W.N.W.; Leh, C.P. Second-generation bioethanol as a sustainable energy source in Malaysia transportation sector: Status, potential and future prospects. Renew. Sustain. Energy Rev. 2011, 15, 4521–4536.
- Venkatesan, P.N.; Sangeetha, D. Characterization and performance study of sulfonated poly ether ether ketone/Fe3O4nano composite membrane as electrolyte for microbial fuel cell. Chem. Eng. J. 2014, 243, 564–571.
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Stor. Mater. 2020, 31, 195–220.
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445.
- Wang, J.; Li, F.; Zhu, F.; Schmidt, O.G. Recent Progress in Micro-Supercapacitor Design, Integration, and Functionalization. Small Methods 2019, 3, 1800367.
- Krauskopf, K.B.; Bird, D.K. Introduction to Geochemistry, 3rd ed.; McGraw-Hill: New York, NY, USA, 2003.
- Logan, B.E. Microbial Fuel Cells; John Wiley-Interscience: Hoboken, NJ, USA, 2008.
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011, 102, 244–252.
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459.
- Sivasankaran, A.; Sangeetha, D. Development of MFC using sulphonated polyether ether ketone (SPEEK) membrane for electricity generation from waste water. Bioresour. Technol. 2011, 102, 11167–11171.
- Sangeetha, D.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells. In Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation, Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–194.
- Hernández-Flores, G.; Andrio, A.; Compañ, V.; Solorza-Feria, O.; Poggi-Varaldo, H.M. Synthesis and characterizacion of organic agar-based membranes for microbial fuel cells. J. Power Sources 2019, 435, 226772.
- Scott, K. Membranes and Separators for Microbial Fuel Cells. In Microbial Electrochemical and Fuel Cells: Fundamentals and Applications; Elsevier Ltd.: Newcastle upon Tyne, UK, 2016; pp. 153–178.
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587.
- Sun, C.-Y.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752.
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 1675–1686.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192.
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344.
- Grzebyk, M.; Poźniak, G. Microbial fuel cells (MFCs) with interpolymer cation exchange membranes. Sep. Purif. Technol. 2005, 41, 321–328.
- Pant, D.; Bogaert, G.V.; Smet, M.D.; Diels, L.; Vanbroekhoven, K. Use of novel permeable membrane and air cathode in acetate microbial fuel cells. Electrochim. Acta 2010, 55, 7710–7716.
- Lefebvre, O.; Shen, Y.; Tang, Z.; Uzabiaga, A.; Chang, I.S.; Ng, H.Y. A comparison of membranes and enrichment strategies for microbial fuel cells. Bioresour. Technol. 2011, 102, 6291–6294.
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696.
- Watson, V.J.; Saito, T.; Hickner, M.A.; Logan, B.E. Polymer coatings as separator layers for microbial fuel cell cathodes. J. Power Sources 2011, 196, 3009–3014.
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Yaakob, Z.; Daud, W.R.W. New generation of carbon nanocomposite proton exchange membranes in microbial fuel cell systems. Chem. Eng. J. 2012, 184, 82–89.
- Choi, T.H.; Won, Y.-B.; Lee, J.-W.; Shin, D.W.; Lee, Y.M.; Kim, M.; Park, H.B. Electrochemical performance of microbial fuel cells based on disulfonated poly(arylene ether sulfone) membranes. J. Power Sources 2012, 220, 269–279.
- Kim, Y.; Shin, S.H.; Chang, I.S.; Moon, S.H. Characterization of uncharged and sulfonated porous poly(vinylidene fluoride) membranes and their performance in microbial fuel cells. J. Membr. Sci. 2014, 463, 205–214.
- Harnisch, F.; Warmbier, R.; Schneider, R.; Schröder, U. Modeling the ion transfer and polarization of ion exchange membranes in bioelectrochemical systems. Bioelectrochemistry 2009, 75, 136–141.
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaroe, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 133–138.
- Vélez-Pérez, L.S.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; López-Díaz, J.A. Industrial acid mine drainage and municipal wastewater co-treatment by dual-chamber microbial fuel cells. Int. J. Hydrogen Energy 2020, 45, 13757–13766.
- Du, Z.; Li, Q.; Tong, M.; Li, S.; Li, H. Electricity generation using membrane-less microbial fuel cell during wastewater treatment. Chin. J. Chem. Eng. 2008, 16, 772–777.
- Du, F.; Xie, B.; Dong, W.; Jia, B.; Dong, K.; Liu, H. Continuous flowing membraneless microbial fuel cells with separated electrode chambers. Bioresour. Technol. 2011, 102, 8914–8920.
- Zhang, X.; Cheng, S.; Huang, X.; Logan, B.E. Improved performance of single-chamber microbial fuel cells through control of membrane deformation. Biosens. Bioelectron. 2009, 25, 1825–1828.
- Rozendal, R.A.; Hamelers, H.V.V.; Guisman, C.J.N. Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 2006, 40, 5206–5211.
- Kim, J.R.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009.
- Santoro, C.; Salar Garcia, M.J.; Walter, X.A.; You, J.; Theodosiou, P.; Gajda, I.; Ieropoulos, I. Urine in bioelectrochemical systems: An overall review. ChemElectroChem 2020, 7, 1312–1331.
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046.
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885.
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process. Biochem. 2004, 39, 1007–1012.
- Walter, X.A.; Greenman, J.; Ieropoulos, I. Microbial fuel cells directly powering a microcomputer. J. Power Sources 2020, 446, 227–328.
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Greenman, J.; Ieropoulos, I. Urine-activated origami microbial fuel cells to signal proof of life. J. Mater. Chem. A 2015, 3, 7058–7065.
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Stinchcombe, A.; Walter, X.A.; Greenman, J.; Ieropoulos, I. Fade to Green: A Biodegradable Stack of Microbial Fuel Cells. ChemSusChem 2015, 8, 2705–2712.
- Kim, C.; Lee, C.R.; Song, Y.E.; Heo, J.; Choi, S.M.; Lim, D.-H.; Cho, J.; Park, C.; Jang, M.; Kim, J.R. Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem. Eng. J. 2017, 328, 703–707.
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biot. 2009, 85, 1665–1671.
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493.
- Tartakovsky, B.; Guiot, S.R. A comparison of air and hydrogen peroxide oxygenated microbial fuel cell reactors. Biotechnol. Prog. 2006, 22, 241–246.
- Harnisch, F.; Schröder, U. Selectivity versus mobility: Separation of anode and cathode in Microbial bioelectrochemical systems. ChemSusChem 2009, 2, 921–926.
