Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Integrative & Complementary Medicine
|
Immunology
Immunotherapy is a novel anti-cancer method which employs a different mechanism to conventional treatment. It has become a significant strategy because it provides a better or an alternative option for cancer patients. The development of immunotherapy should focus on the discovery of biomarkers to screen suitable patients, new targets on tumors, neoadjuvant immunotherapy and the combination of immunotherapy with conventional therapeutic methods.
- pancreatic cancer
- immunotherapy
- immune checkpoint
- myeloid cell
- stroma cell
1. Introduction
Immunotherapy is an anti-cancer method employing a mechanism that is significantly different from traditional therapeutics. It has become an important strategy for the clinical treatment of cancers. Approval of immunotherapeutic drugs has been increasing, with various treatments in clinical and preclinical development [1]. The principle of these drugs for immunotherapy includes the examination of one’s own immune system, the engineering/reeducation of T cells to recognize cancer cells and further to attack them or the adding of inhibitors to block T cell receptors/tumor cell ligands. Immunotherapy can be classified into active immunotherapy, passive immunotherapy and combined immunotherapy. Active immunotherapy directly induces the autoimmune system so that it can recognize specific antigens on cancer cells and attack tumors. Passive immunotherapy uses exogenous substances to exert anti-tumor effects, including monoclonal antibodies, lymphocytes, cytokines, etc. Combined immunotherapy is the combined use of active/passive immunotherapy and traditional therapeutics.
The immune checkpoint is a group of membrane proteins (receptors) expressing on effector cells (e.g., T cells, B cells, NK cells), consisting of multiple co-inhibitory and co-stimulatory pathways. It participates in the elimination of unwanted substances while ensuring self-tolerance, which plays an important role in immunomodulation. Tumor cells containing specific ligands are often able to bind to specific receptors to activate inhibitory checkpoint pathways and evade immune responses. The immune checkpoint executes a regulatory mechanism which in healthy people makes the immune function of T cells maintain a normal and balanced state by regulating the action of ligands and receptors. When T cells are activated, they will express more immune checkpoint receptors, such as programmed cell death protein 1 (PD-1) or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) [2,3]. When these receptors bind to inhibitory ligands, the activity of T cells will be inhibited to avoid excessive immune responses that may damage normal cells and healthy tissues.
Cancer cells have many neoantigens due to many kinds of mutations. In theory, these neoantigens should be recognized by the immune system and activate T cells to destroy cancer cells. However, cancer cells continue to survive and proliferate, indicating that cancer cells can escape the surveillance of the immune system. Most cancer cells producing neoantigens can really be eliminated by T cells and only some cancer cells are capable of avoiding the host immune system. Recent studies have shown that cancer cells can use the mechanism of immune checkpoints to attenuate the activity of T cells [2]. For example, lung cancer cells can express more programmed cell death protein ligand 1 (PD-L1) and binds to PD-1 receptors to inhibit the immune function of T cells. However, the antitumor activity of T cells will be initiated if the inhibitors for PD-L1 or PD-1 bind to the PD-L1 ligand or PD-1 receptor, respectively (Figure 1). A similar inhibition reaction is also found in CTLA-4 receptors on T cells, and other potential targets, such as B and T lymphocyte attenuator (BTLAs) [4], the variable domain immunoglobin suppressor of T cell activation (VISTA), the T cell immunoglobulin and mucin-containing protein 3 (TIM3), the lymphocyte-activated gene-3 (LAG-3, CD223) and CD47 [5]. Additionally, there are agonists of costimulatory molecules to enhance the immune checkpoint signaling in the tumor microenvironment, such as 4-1BB (CD137), OX40 (a member of the tumor necrosis factor receptor superfamily 4, CD134), glucocorticoid-induced tumor necrosis factor receptor (GITR, a type I transmembrane protein), inducible T cell costimulator (ICOS), CD40 and CD28 [6]. Based on these mechanisms, immune checkpoint inhibitors show promise to be developed as drugs for immunotherapy, and there have been many immune checkpoint inhibitors approved by the United States Food and Drug Administration (U.S. FDA) for the treatment of cancer (Table 1).
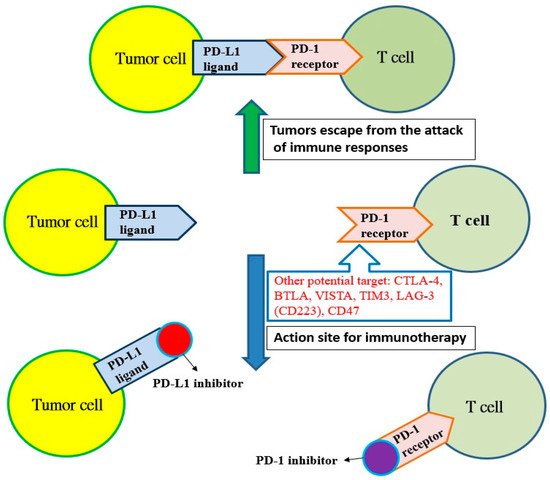
Figure 1. The programmed cell death protein ligand 1 (PD-L1) of tumor cells binds with the programmed cell death protein 1 (PD-1) receptor on T cells, and tumors escape from the attack of immune responses. However, T cells can recognize tumor cells and initiate immunotherapy if the PD-1 receptor is blocked by the PD-1 inhibitor or the PD-1 ligand is blocked with the PD-L1 inhibitor. Other potential targets: cytotoxic T lymphocyte-associated antigen 4 (CTLA-4); B and T lymphocyte attenuator (BTLA); variable domain immunoglobin suppressor of T cell activation (VISTA); T cell immunoglobulin and mucin-containing protein 3 (TIM3); lymphocyte-activated gene-3 (LAG-3, CD223); CD47.
Table 1. The monoclonal antibodies approved by the U.S. FDA to be used as immune checkpoint inhibitor for immunotherapy related to lung cancer or colorectal cancer.
| Immune Checkpoint Inhibitors | Mechanism | Indication |
|---|---|---|
| Pembrolizumab (Keytruda®) | Inhibition of programmed cell death protein (PD-1) | Lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, colorectal cancer, |
| Nivolumab (Opdivo®) | Inhibition of PD-1 | melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colonrectal cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer and esophageal or gastroesophageal junction cancer. |
| Atezolizumab (Tecentriq®) | Inhibition of programmed cell death protein ligand 1 (PD-L1) | Urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC) and hepatocellular carcinoma (HCC). |
| Durvalumab (Imfinzi®) | Inhibition of PD-L1 | Certain types of bladder cancr, lung cancer. |
2. Immunotherapy of Pancreatic Cancer
The pancreas has the function of exocrine and endocrine glands. It secretes pancreatic juice (containing enzymes) and hormones which are needed for digestion and maintaining carbohydrate and growth balance in the body. The preliminary statistics show that pancreatic cancer caused by cancer cells in the pancreatic islets accounted for about 5–10% of cases, while pancreatic duct adenocarcinoma accounted for about 90–95% [48,49,50]. Compared with other cancers (e.g., lung cancer, colorectal cancer, breast cancer, cervical cancer, etc.), pancreatic cancer has lower incidence but up to 85% of patients are clinically diagnosed at advanced stages and cannot undergo surgery [51,52]. Even if there is a chance for surgery, many patients will still relapse, and the diagnosis is almost equivalent to a declaration of death. Consequently, pancreatic cancer is referred as the “King of Cancer” because of the difficulty of detecting it early on and because the efficacy of treatment is poor and the recurrence rate high [51,52,53]. At present, the most effective method for pancreatic cancer treatment is surgical removal, but only about 15% of patients are clinically suitable for surgery. For patients who are unable to undergo surgery, only chemotherapy, radiotherapy, targeted therapy or combined therapy can be used to relieve the disease and prolong the survival period, but the efficacy is usually not ideal [53,54,55,56].
Immunotherapy is another option for pancreatic cancer patients who respond poorly to conventional methods. There have been many clinical trials trying to evaluate the effectiveness of immunotherapy for pancreatic cancer; however, current studies have failed to change the clinical outcome significantly. In addition, the identification of pancreatic tumor-associated antigens, which functionally contribute to pancreas pathogenesis, and their successful implication in cancer treatment is still challenging. Fortunately, mucin 4 (MUC4), a glycoprotein with a high molecular weight, seems to be a novel and attractive tumor-associated antigen, because it is overexpressed in mouse and human pancreatic tumors, not being detected in the normal pancreas [57]. The recombinant MUC4 domain and predicted immunogenic T cell epitopes can induce cell-mediated and humoral immune responses against MUC4, suggesting its potential to be used as a vaccine candidate for the treatment of pancreatic cancer. Additionally, immunotherapy potentially has the synergistic effect of increasing the response rate and combining with other conventional therapies [56,58]. Several agents for single use for immunotherapy are under study as follows.
2.1. Immune Check Point Inhibitor
Immune checkpoint blockers (e.g., anti-CTLA-4, anti-PD-1, anti-PD-L1) have shown curative effects in some malignant tumors, but no clinical trials have proved any efficacy in most cases of pancreatic cancer at stages I and II. However, a combination therapy combining immune checkpoint inhibitors and radiotherapy and/or chemotherapy has initially shown positive results [59,60,61].
Group 2 innate lymphoid cells (ILC2s) found in cancers of mammal tissues is known to be able to modulate inflammation and immunity, but the role of ILC2 in cancer immunity and immunotherapy is still poorly understood. Moral et al. demonstrated that tissue-specific tumor immunity was activated by ILC2s infiltrated from pancreatic ductal adenocarcinomas [62]. In animal study, they found that interleukin-33 (IL33) activates tumor ILC2s and CD8+ T cells to limit pancreas-specific tumor growth in orthotopic pancreatic tumors but not heterotopic skin tumors [62]. The tumor ILC2s express the inhibitory checkpoint receptor PD-1. The PD-1 blockade decreases ILC2 cell-intrinsic PD-1 inhibition to expand tumor ILC2s and increase immune responses to control tumor growth, indicating that activated tumor ILC2s may be targets of anti-PD-1 immunotherapy. The results showed that ILC2s are anti-cancer immune cells for pancreatic cancer immunotherapy because they can be used as tissue-specific enhancers that amplify anti-PD-1 efficacy [62]. The immunotherapy strategy to collectively target anti-cancer ILC2s and T cells is potentially applicable in the treatment of pancreatic cancer.
2.2. Therapeutic Cancer Vaccine
Therapeutic cancer vaccines (e.g., whole cells, dendritic cells, DNA and peptide vaccines) can stimulate the presentation of immunogenic cancer antigens to the immune system. A vaccine using a single peptide derived from the tumor-associated self-antigen human telomerase demonstrated no response or survival benefit in patients with metastatic pancreatic cancer in a phase III trial [63]. However, these vaccines have the potential to activate cancer antigen-specific cytotoxic T lymphocytes and trigger subsequent anti-cancer immune responses [59,60,63].
Tumor-derived exosomes (TEXs) are lipid nanoparticle encapsulated vesicles that transport bioactive substances into the microenvironment to promote tumor progression. Nonetheless, many recent studies show that TEXs can efficiently enhance immune responses against tumors if they are given appropriately [64]. Naseri et al. summarized in a review the potency of TEXs in inducing effective anti-tumor responses in vitro and preclinical studies [64]. The recently modified strategies further improve TEX vaccination efficacy. Although it is required for TEXs to have further experimental studies to determine efficacy and side effects, they indicated that TEXs are promising to become new objects in cancer vaccination based on tumor antigen-selective high immunogenicity [64]. There may be a little hope that TEXs will prove a breakthrough in tumor immunotherapy.
2.3. Adoptive Cell Transfer
The patient’s own tumor antigen-specific T cells were collected and genetically modified. These cells were cultivated and proliferated in vitro, and then re-transplanted into the patient’s body to enhance immunity and improve immune responses. Engineered chimeric antigen receptor T (CAR-T) cell therapy is clinically the most common type of adoptive cell transfer therapy [59,60]. Unfortunately, translating CART therapy to malignancies is still challenging, because it is difficult to identify a safe, specific and homogeneously expressed target [65]. However, several self-antigens, such as carcinoembryonic antigen, prostate stem cell antigen, mesothelin and human epidermal growth factor receptor 2, are significantly overexpressed in pancreatic duct adenocarcinomas and are related to worse prognoses [66], and therefore likely to be prospective targets.
2.4. Agonistic Immunotherapy
Antigen presentation cell (APC) activators and T cell activators are two methods under investigation. It is known that CD40 targets modulate the activation of APC and finally result in T cell activation (Figure 2). Agonists of costimulatory molecules like CD40 have shown promising results in preclinical studies and are currently being tested in ongoing clinical trials [67]. Activating CD40 therapy can mimic the assistance of T cells and allow APCs to be more competent to effectively present antigens to T cells and activate them. No enhancement of long-term survival was observed in phase I clinical trials, suggesting the therapy did not induce immune memory [68]. However, the combination of activating CD40 monotherapy and gemcitabine can activate macrophages to kill cancer cells and has shown efficacy in the clinical trial [59,60].
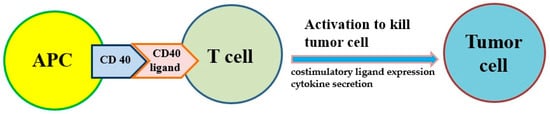
Figure 2. The costimulatory receptor CD40 on antigen-presenting cells (APCs) can improve the antitumor response of T cells because it induces costimulatory ligand expression and cytokine secretion that drive antitumor activity.
2.5. Myeloid-Based Immunotherapy
Several subtypes of myeloid cells derived from bone marrow (e.g., macrophages, dendritic cells, neutrophils, monocytes and granulocytes, etc.) significantly regulate the growth and progression of tumors via the supplement of tumor-promoting factors and molecules that suppress CD8+ cytotoxic T cells [69]. Macrophages and monocytes are considered the most populous myeloid lineage cells in developing solid tumors (Figure 3) and play a crucial role in regulating both protumor and antitumor immune responses. Targeting of these cells potentially attenuates solid tumor progression by the induction and mobilization of cytotoxic T cells [69]. The abnormal immune response of pancreatic cancer is partly regulated by immunosuppressive bone marrow; suppressing the bone marrow can suppress the tumor. The bone marrow is controlled by cytokines, chemokines and signaling molecules. The receptors of cytokines, chemokines and signaling molecules can establish the immunosuppressive tumor microenvironment and potentially be used as therapeutic targets [59,60].
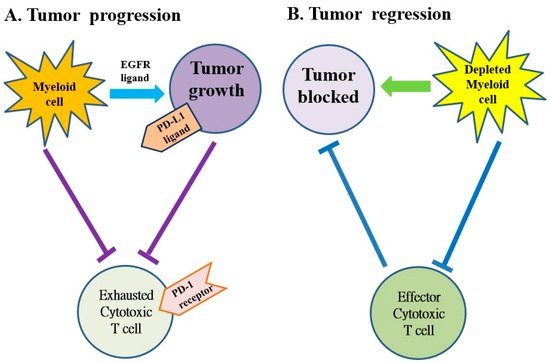
Figure 3. The myeloid cells protect tumor cell viability by blocking the anti-tumor responses of cytotoxic T cells in pancreatic cancer. (A) The myeloid cells block anti-tumor immune responses of cytotoxic T cells by activating the programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1) checkpoint. (B) The myeloid cell depletion reverses immune suppression and activates CD8+ T cells to block the growth of tumors. EGFR: epidermal growth factor receptor.
2.6. Stroma-Modulating Immunotherapy
The tumor microenvironment is the environment surrounding the tumor, including blood vessels, immune cells, fibroblasts, signaling molecules and the extracellular matrix [71,72]. Tumors and the microenvironment are so closely related that tumors can influence the microenvironment via the release of extracellular signals, the promotion of tumorigenesis and the triggering of neighboring immune tolerance (Figure 4). The immune cells in the microenvironment can affect the growth and evolution of cancer cells and tumorigenesis is regulated by the microenvironment [71,72]. The proliferative matrix of pancreatic cancer is a key component of the immunosuppressive tumor microenvironment and an obstacle to effective treatment. Although it is still controversial whether targeting of the matrix is beneficial for patients, early-stage studies proving the therapeutic potential of modulating substrates have already begun [54,55,73].
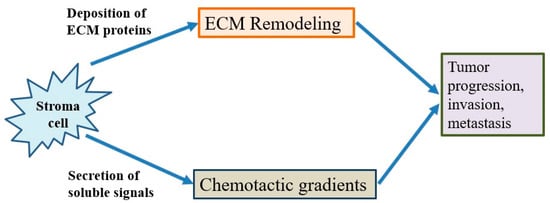
Figure 4. The behavior of cancer cells is affected by their environment. The stromal cells are able to release chemotactic growth factors, and cell-induced mechanical strains are able to rearrange extracellular matrix (ECM) fibers. These factors are correlated with tumor progression, invasion and metastasis. In addition, the tumor cell interacts with fibroblasts to lead to the deposition of new ECM proteins, and physical forces from strains are related with fiber alignment, resulting in persistent migration and invasion of cancer cells.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222312836
This entry is offline, you can click here to edit this entry!
