Extracellular vesicles (EVs) are 50–1000 nm vesicles secreted by virtually any cell type in the body. They are expected to transfer information from one cell or tissue to another in a short- or long-distance way. RNA naturally present in EVs might be limited in a physiological context.
- extracellular vesicles
- exosome
- RNA
- miRNA
1. Introduction
2. RNA-Based Mechanism of Action for Native EVs
-
In glioblastoma (GBM), Abels et al. describe short distance communication through EVs from tumor cells to microglia to induce microglia reprogramming. The presence of EVs was detected in 0.3% of microglial cells, and the presence of the miRNA of interest transferred by EVs was detected in these sorted 0.3% of cells. However, no clear target protein silencing was found (only 4 out of 59 validated targets). It may be possible that the miRNA detected would partly be coming from the retention of EVs (and its associated miRNA) in endosome of microglial cells, without intracytosolic delivery [19].
-
Lucero et al. demonstrate the short distance effect of glioblastoma-derived EVs to induce angiogenesis via miRNAs in vitro, and claim it to be also valid in humans only based on a correlation with a human glioblastoma transcriptomic “fingerprint” [20]. However, no clear demonstration of causality is proposed.
-
Shen et al. demonstrate the effect of EVs derived from tumors to induce stemness via miRNA in surrounding cells in vitro (at supra physiologic doses) and claim it to be also valid in vivo in tumor-bearing mice. However, they used Rab7 KO tumors as a control to inhibit EV production, a KO that also has a lot of other side effects [21]. It is therefore difficult to know whether this effect is mediated by EVs and by the miRNA inside them.
-
Ying et al. demonstrate a role for miR-155 transferred by EVs in vitro in glucose tolerance and use an elegant system of bone marrow transplantation to investigate the role of hematopoietic derived miR-155 in a KO mouse. They later claim that the partial rescue of physiologic glucose tolerance is mediated by EVs in vivo although it may also be mediated by other intercellular transfer mechanisms like tunneling nanotubes (TNT), especially to transfer at short distance miRNA from a very macrophage-rich organ like liver to surrounding hepatocytes [22]. The same miRNA-155 has indeed been shown to be able to be transferred through TNT [23].
-
Chen et al. claimed that miR-375 overexpressing EVs were able to promote bone regeneration but the effect in vivo is not significantly different from the EV control group [5].
-
Thomou et al. help us to raise other non-trivial questions on vesicular versus non vesicular mediated RNA transfer. He proposed that EVs from adipose tissue would be able to transfer miRNA to liver cells and induce RNA silencing in vivo. The protein expression is reduced by up to ∼95% after injection of serum-derived EVs (from donor mice with brown adipose tissue expressing the miRNA of interest) to miR-KO mice [24]. Strictly speaking, the demonstration proves that a serum factor purified with common EV purification protocols from the donor mice leads to specific miRNA-mediated silencing in mice. It raises the question of whether this effect may be at least partly mediated by an extra-vesicular miRNA in serum co-purified with EVs.
-
Other teams claimed the demonstration of an efficient transfer of CRE-mRNA via EVs [27,28,29] in vivo. This highly sensitive “on/off” system induces or stops the expression of a particular fluorescent protein upon delivery of the CRE-recombinase protein or its RNA. Although it is very different from a physiologic system, it may still be of interest as a proof of concept. However, this assay has shown limited transduction efficacy even with a high dose of EVs (e.g., in Ilahibaks et al. [30] achieved ∼15% transduction efficacy by ∼8300 EV/cell in vitro, i.e., intra-cytosolic transfer of at least one CRE protein or RNA). More importantly, it may be biased by the transfer of a single CRE recombinase protein (instead of CRE-mRNA) from the donor EVs, although it was not detected in these articles. On the contrary other teams clearly reported the presence of CRE protein in EVs produced from CRE-producing cells [31].
3. Physiological Effect of RNA Cargo in EVs: A Natural RNA Vector?
3.1. Stochiometric Evaluation of RNA Loading in EVs
Sverdlov claimed that it is very unlikely that naturally circulating EVs transfer a significant part of information through RNA in vivo at long distances in physiological states [14]. He argued that the best candidates for information transfer would be self-amplifying (e.g., mRNA) and/or have a regulatory function (e.g., a transcription factor, a miRNA). At the time, he made the hypothesis that RNA inside EVs was not subject to strong selection. Baglio et al. [38] and other groups found that most RNA in various types of EVs (from tumor, MSCs, immune cells and serum, isolated by various methods (ultra)-centrifugation or affinity column) were small <400 nucleotides (nt) long RNA [39,40,41,42]. Among them, most are tRNAs (that can hardly be expected to have an effect) and miRNA only constituted ∼0.9% [43] of RNA reads. Although the miRNA are relatively enriched (∼10 fold compared to cell RNA4), enrichment may largely be due to the nonspecific size selection biased to the smaller sizes such as tRNAs. As an example, 16 S RNA (1,6 kB), a typical medium-size RNA has a hydrodynamic diameter of ∼30 nm [44], whereas miRNA (20–83 nts) have a cylinder shape with a 2 nm diameter and a 7–20 nm length. mRNA encapsulation inside EVs also depends on their local concentration around EV formation sites, as well as mRNA interaction with membrane lipids and proteins [45]. Before being functional, miRNA are getting through the pri- and pre-miRNA state. To be potentially active if they get to the target cell cytosol, miRNA needs either (i) to be not yet associated with Ago2 to form the RISC complex but still able to bind to it (i.e., being pri- or pre-miRNA) and therefore they would be able to bind it later on in the recipient cell cytosol or (ii) to already be associated with the RISC complex as a miRNA, a state in which they can exert their silencing activity directly. Importantly, association of miRNA to the RISC complex allows them to be much more stable than if left alone where it can be rapidly degraded by nuclease, in particular in the context of EV travel through endosomes (containing nucleases) in the target cell.
Sverdlov proposed a rough approximation of the maximal amount of RNA per EV if they are densely packed in EV of 100 nm diameter: ∼1600 RNA/EV for 1000-nt RNA and ∼6700 RNAs/EVs for 200 nts RNA. However, when measured by total RNA quantification [48], the number of RNA per EV was less than one in serum-derived EVs. Another team reported the presence of ∼7 µg of RNA per 1010 EVs dosed by bulk representing ∼6500 RNA molecules per EV [43], but the presence, as discussed by the authors, of contaminating surrounding extra-vesicular RNA may artificially enhance this number. As an example, once extra-vesicular RNA is removed from serum-derived EV preparations (using differential centrifugation and size-exclusion chromatography) only ∼2.5% of total miRNA remains in the serum-derived EV fraction [25,26,49,50,51,52]. Most of the time, purification strategies used are not allowing complete extra-vesicular RNA removal (in particular in serum where it represents a large fraction of RNA), therefore attribution of a particular effect to intra-vesicular EVs may be difficult. Quantitative results on the amount of miRNA per EVs estimates that most represented miRNA can hardly be found in 1 out of 100 exosomes (the range varies for each miRNA from one copy per 9 exosomes to one copy per 47,162 exosomes, mean of 1 copy per 121 exosomes using digital PCR, a reliable and sensitive quantitative method) [25]. Knowing that they detected 131 miRNA in total, the estimated miRNA per EV should be considered to be ∼1 per EV.
3.2. Navigating the Bloodstream and Getting to the Target?
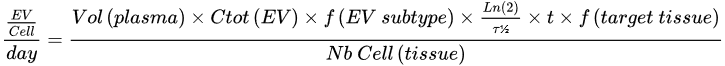
3.3. A Very Interesting Intra-Cytosolic RNA Delivery (Endosomal Escape)
3.4. Is the Physiologic RNA in EVs Dose Sufficient to Achieve an Effect?
The RNA-based mechanism of action (MOA) for effects mediated by non-modified native EVs in therapeutic conditions has previously been challenged by comparing it to data obtained from siRNA experiments. In most preclinical studies, EV doses usually range from ∼1 to 200 µg per mouse [81], corresponding to about 1010 to 1012 EV/mouse depending on EV preparation and dosage methods. If we consider ∼1 miRNA per EV, this dose represents ∼1010 to 1012 miRNA per dose, corresponding to about ∼0.2–20 ng of miRNA/mouse or ∼0.016–1.6 pmol/mouse. siRNA doses reported to be efficient in vivo in systemic injections are rather in the microgram range (27 to 750 µg/mouse [82,83]). One explanation is that the observed therapeutic effect of native EVs is not mediated by their naturally loaded (mi)RNAs. Indeed, this ∼103–104 fold difference was though too big to be explained by a very high difference in delivery efficacy [43,80]. However, this may be now discussed in view of recent results comparing engineered EVs to synthetic RNA nanovectors.
Indeed, recently reported delivery efficacy of EVs obtained in vivo show a ∼10–300 fold improvement in favor of EVs [84] compared to lipid nanoparticles (although the authors discuss the estimation of miRNA concentration with their method may favor EV reported efficacy by ∼10 fold [85,86]). The authors used the natural ability of pre-miR-451 to be enriched preferentially in EVs and used it as a backbone to couple with an siRNA of interest in order to target it inside EVs [84]. They then used these engineered EVs to target the liver, intestine or kidney glomeruli and achieve various target knockdown. Interestingly, this ∼10 to-300 fold improvement in terms of RNA cytosolic delivery in favor of EV in vivo is fully consistent with independent data on delivery efficacies reported for synthetic vectors: EVs reach a ∼20% endosomal escape rate [71] compared to 0.1 to 2% for synthetic vectors [87], which leads to a ∼50 fold increased cytosolic delivery. Even higher differences (up to 104) were reported in the delivery efficacy in favor of EVs in vitro [32]. Importantly, such a fold change also takes into account the very different endocytosis rate that favors EVs compared and synthetic vectors in vitro but not in vivo [88].
Altogether, these quantitative estimates show (Figure 1) that distant communication by EVs via RNAs probably has limited efficacy in physiological conditions, although it may be a bit different in pathological conditions and in the therapeutic use of EVs that are engineered to load large amounts of specific RNA.
4. Considerations on RNA Based Information Transfer in Therapeutic Settings
4.1. Considerations on the Therapeutic Effect of RNA from Unmodified EVs
4.2. Considerations on the Effect of RNA from Engineered EVs
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13111931
