Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Endoscopic ultrasound is a reliable diagnostic and therapeutic method that has an established role, foremost in pancreatobiliary pathology.
- EUS
- endoscopic ultrasound
- chronic liver disease
1. Introduction
At the beginning of the 21st century, chronic liver disease (CLD) is a significant public health concern. It has been reported that globally 1.5 billion persons had CLD in 2017, most commonly resulting from non-alcoholic fatty liver disease (NAFLD) [1]. Moreover, the burden of NAFLD and alcohol-related liver disease (ALD) is expected to increase over the coming years [2]. Noninvasive diagnostic modalities developed in recent years have greatly advanced the evaluation of CLD, but there are still many clinical situations where accurate diagnosis and staging depend on histopathology. Endoscopic ultrasound (EUS) is an unavoidable method for the evaluation of the pancreatobiliary and upper gastrointestinal tract with an expanding role in the field of hepatology. The limitations of conventional diagnostic tools and percutaneous interventions for liver disease, mostly done with transabdominal ultrasound (US) or computed tomography (CT) guidance, have made EUS an attractive alternative, predominantly due to the enhanced imaging quality and safety profile along with the biopsy acquisition ability regardless of body habitus [3][4].
2. EUS-Guided Liver Biopsy (EUS-LB)
In recent decades, there have been many advances in noninvasive diagnosis and investigation of liver diseases, but LB remains the best means for obtaining and clarifying the underlying pathology, determining the severity of liver damage, monitoring disease progression, or supporting research [5][6]. The most important issue regarding the procedure is to obtain an adequate liver specimen, which will allow detailed histopathological interpretation. The American Association for the Study of Liver Diseases guidelines suggests that adequate LB specimens contain a tissue core of at least 2–3 cm in length with the presence of more than 11 complete portal tracts [6]. Percutaneous LB continues to be the most utilized technique for histopathological assessment of liver tissue, which uses an image-guided approach (US or CT) to reduce the complication rate. Transjugular biopsy accesses the liver through the superior vena cava and the hepatic vein, without traversing the liver capsule, which is useful in patients with bleeding diathesis, presence of ascites and morbid obesity, or in those who could benefit from simultaneous direct measurement of the hepatic venous pressure gradient (HVPG) [7]. Major complications following traditional methods of LB reach up to 2.5%, the most common being hemorrhage and pain [8][9], while mortality rates occur at 0.2% [10]. Further, the heterogeneity of liver fibrosis may contribute to sampling variability, which has been recognized as a potential pitfall of standard LB techniques [6][11].
Since the first published cases of EUS-LB in 2007 using a novel Tru-Cut core biopsy needle (QuickCore; Cook Medical, Winston Salem, NC, USA) [12][13][14], there have been numerous studies showing comparable adequacy and complication rates [3]. Yet, the EUS technique still affords many advantages over percutaneous and transjugular approaches. Due to the proximity of the ultrasound device to the liver, EUS allows for a detailed view of a patient’s anatomy in real-time and the avoidance of other structures, including the adjacent vasculature and major bile ducts, thus reducing procedure-related complications [15]. In this way, multiple cores from both right and left liver lobes can be obtained, increasing the adequacy and yield of tissue [16]. Additionally, EUS-LB is performed with either conscious sedation or under anesthesia, significantly improving patient tolerance and comfort [17][18]. The procedure is quick, adding only a few minutes to the overall procedure time [19]. We often perform EUS for the evaluation of elevated liver enzymes in patients with a dilated common bile duct, and in case of non-diagnostic findings, patients can undergo EUS-LB in the same session, which is likely to reduce overall time, cost of multiple procedures, and expedite clinical management [16]. Finally, EUS-LB has a shorter average recovery time compared to conventional LB methods [18].
The first meta-analysis performed on this topic included nine studies from 2009 to 2016, which demonstrated that EUS-LB has a similar diagnostic yield (93.9% (95% confidence interval [CI], 84.9–97.7)) and adverse event rates (2.3% (95% CI; 1.1–4.8); the pooled rate of bleeding 1.2%) when compared to data from studies of percutaneous and transjugular approaches [20]. The subgroup analyses based on the needle type (core needle (QuickCore and ProCore, Cook Medical, Bloomington, USA) vs. fine-needle aspiration (FNA) needle, all 19 G) showed that the FNA needle had a significantly lower rate of achieving insufficient specimens than core biopsy needles (4% vs. 20%, p = 0.03). The possible explanation for this lies in the fact that the majority of inadequate specimens were associated with the use of the QuickCore biopsy needle, which is no longer commercially available [21]. In one study comparing the QuickCore and Procore needles for EUS-LB, the QuickCore was significantly inferior in terms of obtaining a histologic diagnosis (73% vs. 97%), number of complete portal triads (CPT), and aggregate specimen length [22]. In the past several years, multiple dedicated EUS-guided fine-needle biopsy (FNB) devices with enhanced tip designs for maximal tissue acquisition have been made available for commercial use. Schulman et al. tested four EUS needle types against two 18G percutaneous needles on human cadaveric tissue (19G FNB SharkCore, Medtronic, Sunnyvale, CA, USA; 19G FNA Expect, Boston Scientific, Natick, MA, USA; 19G FNB Echo Tip HD ProCore, Cook Medical Inc., Bloomington, IN, USA; 22G FNB SharkCore, Medtronic, Sunnyvale, CA, USA) and reported that the novel 19G FNB needle was associated with the maximal number of CPTs. Moreover, a 22G FNB needle was not statistically different from an 18G percutaneous needle [23]. When comparing 22G FNB versus 19G FNA needles, tissue adequacy is higher for the 19G FNAs (88% vs. 68%, p = 0.03), mainly because samples obtained from a smaller caliber needle are more prone to fragmentation during specimen processing [24]. Specimen fragmentation remains a significant limitation of EUS-LB because it can significantly compromise diagnostic accuracy [25]. Finally, recent data suggest that EUS-LB with a 19 G FNB needle provides better histologic specimens than does the technique in which FNA needles are used [26] (Figure 1 and Figure 2).
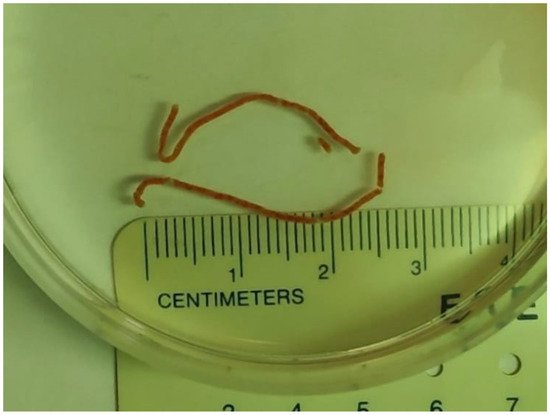
Figure 1. Two visible liver core specimens following EUS-LB.
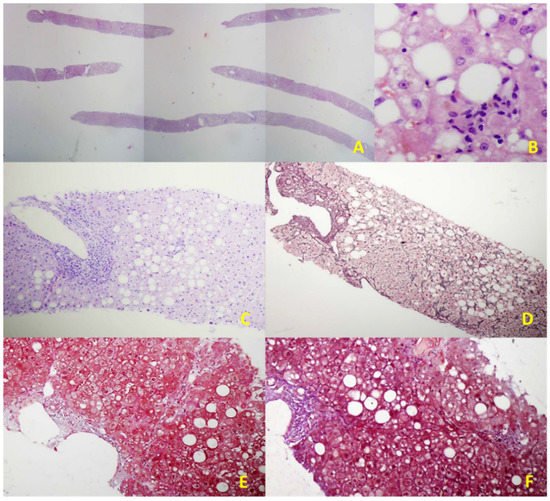
Figure 2. Illustrative histological features of NAFLD in an EUS-LB specimen. (A) Up to 75 portal triads per liver specimen obtained by EUS-LB using 19-gauge FNB needle (HE staining, magnification 20×); (B) Focal necrosis, macrovesicular steatosis, and ballooning of hepatocytes grade 1. (HE staining, magnification 400×); (C) Mild chronic infiltration in portal tract with the preserved limited plate. Panacinar steatosis (up to 30%); (HE staining, magnification 100×); (D) Moderate, zone 3 perisinusoidal fibrosis (Brunt, fibrosis stage 1b); (Gomori; magnification 100×); (E) Mild, zone 3 perisinusoidal fibrosis (Brunt, fibrosis stage 1a); (Masson; magnification 100×); (F) Bridging fibrosis (Brunt, fibrosis stage 3); (Masson; magnification 100×).
Beyond the needle design and size, there is also the issue of optimal technique to improve the diagnostic yield of EUS-LB. Many endoscopists use suction or slow-pull techniques with FNA. In the human cadaveric study, the type of suction technique did not affect sample adequacy [23]. Hasan et al. used a 22G FNB needle (Acquire; Boston Scientific, Marlborough, MA, USA) and did not apply any suction but developed a technique to limit tissue fragmentation. This technique restricted elevator utilization, and the stylet was slowly re-inserted while keeping the assembly straight to avoid tissue distortion [27]. The wet suction technique, which uses a saline-filled pre-vacuum syringe, showed high effectiveness for EUS-LB, using the 19G Sharkcore or a standard 19G FNA needle even with a single pass and one actuation, as reported in a retrospective study on 165 patients [28]. Furthermore, priming the needle with dilute heparin instead of saline can decrease the formation of blood clots in the needle and improve tissue handling. It has been demonstrated that heparin priming does not lead to bloodier specimens, nor does it increase adverse events of FNA. In a prospective study on 40 patients, using heparin-primed needles improved tissue adequacy compared with dry suction techniques [29]. In a large prospective, multicenter study with a 19G FNA needle, Diehl et al. reported using the fanning technique, a well assessed FNA technique that involves several to-and-fro movements of the needle in the liver with slight variation in the access angle, allowing sampling of new areas of the lobe [19]. The most recent meta-analysis (23 studies, 1326 patients) indicates that using an FNB needle with the slow-pull technique may provide better specimen quality and higher diagnostic yield [30]. Nevertheless, we need more prospective comparative studies to more precisely assess the superiority of various EUS-LB techniques.
There are several limitations to the widespread utilization of this technique. EUS requires a prolonged learning curve to achieve competency [31] in comparison to conventional techniques, which require less technical expertise. Endoscopic equipment and the devices utilized for the procedure are expensive. Conscious sedation or anesthesia further increases the cost, and there are also certain risks in an endoscopic procedure. However, EUS-LB is an evolving technique that already has an important role in settings with relevant expertise, mainly because of the superior control of the operating field, low incidence of adverse events, accessibility of the various parts of the liver, and greater patients’ comfort [32].
3. EUS-Guided Portal Hypertension Measurement
The hepatic venous portal pressure gradient or portal pressure gradient (PPG) reflects the degree of portal hypertension (PH) and is the best prognostic indicator in liver disease [33]. The current standard for evaluation of PH is an indirect measurement of HVPG via right jugular vein access, where the free hepatic venous pressure is recorded and subtracted from the wedged hepatic venous pressure to determine the HVPG. Despite the overall safety profile, the method is highly invasive and requires technical expertise found at specialized medical centers and may not always accurately reproduce true portal venous pressures, especially in patients with pre- and post-hepatic etiologies of PH [4][34]. Direct percutaneous portal vein catheterization is usually avoided because of the high risk for complications [35]. Due to the relative proximity of the portal vein to the tip of the echoendoscope during the EUS exam, this method emerged as an alternative to standard percutaneous routes for obtaining hepatic vascular access (Scheme 1).
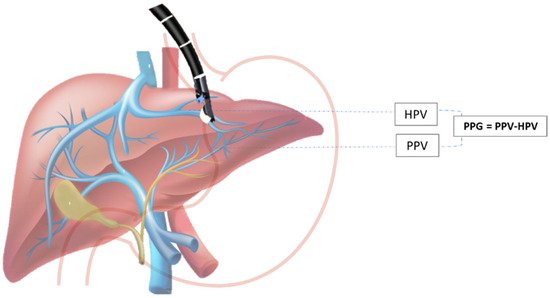
Scheme 1. EUS-guided portal pressure gradient (PPG) measurement. PPG is calculated as the difference between hepatic vein pressure (HVP) and direct portal vein pressure (PVP).
The first human case on EUS-guided PPG measurement (EUS-PPG) was reported in 2014 by Fuji-Lau et al. [36]. Subsequently, the prospective pilot study evaluated the use of EUS-PPG measurement in 28 patients with suspected or confirmed cirrhosis by using a 25 G needle and a compact manometer. One hundred percent technical success and no adverse events were reported [37]. A recent study from Zhang et al. confirmed a high degree of consistency between EUS-PPG using a 22-gauge FNA needle and HVPG in patients with acute and subacute PH. The authors showed a strong association between the two variables, with a Pearson correlation coefficient of 0.923 [38]. Although the current literature suggests that EUS-PPG measurement as a means of direct portal pressure evaluation is safe and feasible, larger clinical trials and comparative studies assessing standard methodology and clinical effectiveness of this method are needed. The fact that this can be a part of a multiprocedural intervention (general endoscopic assessment, variceal screening, and EUS-LB and EUS elastography) that can be done during a single endoscopic exam will presumably affect the acceptance of this method in the future [39].
4. Detection of Varices and Prediction of Variceal Bleeding
Due to the proximity of internal organs to the gastrointestinal tract, one of the main indications of EUS has, so far, been redirected to therapeutic interventions. As a diagnostic tool, EUS has important implications for patients with PH, offering visualization of structures in submucosal spaces, which include varices in the gastrointestinal tract and vascular structures that surround the gastrointestinal wall, and risk evaluation of future bleeding from EGV. The instrument channel in echoendoscopes enables the use of several devices for endoscopic interventions of EGV using glue injection or coil embolization [40].
Variceal hemorrhage is the main cause of upper gastrointestinal bleeding in patients with PH (70% of cases) and represents one of the most serious complications in these patients, with overall six-week mortality at around 15–25% for esophageal varices (EV) and 45% for gastric varices (GV) [41]. According to studies that evaluated the role of EUS in the detection of EV, radial EUS was significantly inferior to standard EGD [4], leaving EGD as an endoscopic method of choice for diagnosis, surveillance, and treatment of EV [42]. However, more recent studies have shown the comparable role of EUS to standard EGD in detecting EV (including small EV), predominantly due to newer and improved technical specifications of echo-endoscopes (smaller tip in echo-endoscope, small water-filled balloons, small 20-Hz ultrasound transducers, high-frequency ultrasound miniature probes, and higher video resolution) [33][43]. EUS can visualize esophageal collateral vessels that can be divided into two main groups: (1) periesophageal collaterals (veins small in size) located close to the esophageal wall; and (2) paraesophageal collaterals (large in size), which are located away from the esophagus [44]. The detection of collateral vasculature that surrounds the esophagus has important clinical implications, predominantly for prognostic purposes. The literature data have shown that the presence of severe collateral and perforation veins detected by EUS can help in the prediction of the recurrence of EV before and after treatment (sclerotherapy or band ligation), suggesting closer follow-up in this subgroup of patients [45][46]. In addition to predicting the risk of variceal recurrence, EUS may also predict the risk of recurrent variceal bleeding after endoscopic variceal ligation (EVL) with sensitivity and specificity around 90% [47]. The most important signs that correlated with higher rates of recurrent variceal bleeding included the diameter of paraesophageal veins and the detection of perforating veins prior to and after endoscopic sclerotherapy, and higher rates of cardiac intramural veins [48][49]. Furthermore, EUS can predict the risk of bleeding by the assessment of the hematocystic spots on the surface of EV (identified as saccular aneurysms), which are closely associated with a high risk of variceal rupture [50]. EV can be eradicated using EUS-guided EVL or sclerotherapy, keeping in mind the abovementioned advantages of EUS in predicting/reducing variceal recurrence. Minor complications after EUS-guided sclerotherapy have been reported, with no significant differences from complications induced by standard EGD [51][52]. A randomized clinical trial that compared standard EGD sclerotherapy and EUS-guided sclerotherapy of the feeding veins to EV showed similar recurrence rates for both groups [52].
5. EUS-Guided Therapy for PH
On the contrary to EV, gastric varices (GV) are present in a smaller proportion of patients with cirrhosis (20%). It is known that a hemorrhage from cardiofundal varices is less frequent but often more severe and not easily controlled, providing a higher risk of recurrent bleeding and mortality (when compared to EV) [41][53]. While standard EGD still represents the gold standard in detecting EV, EUS has better sensitivity in diagnosing GV [54], with a detection rate two times higher [55]. According to some authors, EUS can evaluate ectopic duodenal varices [56], easily distinguish thickened gastric folds from small GV [57], and help in the diagnosis of portal gastropathy, showing diffuse thickening of the gastric wall with dilated paragastric veins (differential diagnosis to “watermelon stomach”) [33]. EUS also has an important role in the characterization of GV, visualization of treatment in progress, and confirmation of obliteration using Doppler [58][59]. Furthermore, EUS can easily measure the size of GV, which directly correlates with their flow volume [60]. Nowadays, the standard endoscopic management of fundal GV in acute bleeding or selective therapy is endoscopic cyanoacrylate (CYA) injection, which can be complicated with fever, chest pain, post-injection ulcers, re-bleeding (15 to 30%), embolic events (the incidence increases with the amount of CYA injected), or death. One of the advantages of EUS includes the identification of GV in the setting of acute bleeding when blood and clots in the gastric lumen disable an adequate endoscopic view [61]. Since the risk factors for re-bleeding include varix size, presence of para-gastric veins [62], and deficiency of complete obliteration of the GV or of the perforating vascular channels, which are unavailable for detection or eradication during standard EGD, a possible therapeutic role of EUS is arising. A retrospective study on 101 patients treated with glue injection after an episode of GV hemorrhage showed significantly lower re-bleeding rates in those patients in whom EUS was aggressively used during follow-up with the intention of achieving a complete obliteration of variceal veins [63]. EUS-guided hemostasis of GV (with different available methods: injection of CYA, coils, coils with CYA injection, thrombin, or coils with an absorbable gelatin sponge (AGS)) allows assessment of the variceal blood flow, selective targeting of the varices with very exact treatment into the lumen of varix or into its feeding vessel (lowering the required dose of adhesive agent), and monitoring of the obliteration results (confirmation of cessation of variceal blood flow using Doppler and the presence of echogenic GV) [64]. Several studies evaluated the role of CYA injection alone, either in primary prophylaxis [65][66] or acute GV bleeding [67][68][69], with an overall GV obliteration rate of 100% for the first group and 77–100% for the second. The re-bleeding rate was 0% and 5%, respectively, and severe complications were detected in the second group and included pulmonary embolism and splenic infarct in 5% of cases. As it was mentioned earlier, EUS-guided CYA injection has a risk of distal embolization (embolization to the pulmonary arteries and systemic embolism), and a multidisciplinary assessment is required to evaluate the potential presence of a septal defect prior to EUS-guided CYA injection. Furthermore, EUS-guided coil injection (either with or without CYA injection that can be delivered using standard 22- or 19-gauge needles used for FNA) may be a future method of choice to reduce the risk of embolization due to providing primary hemostasis [70][71]. In the literature, limited data showing the role of coil injection alone in treating GV are available. Five studies evaluated coil injection in primary prophylaxis [67][72], both primary prophylaxis and acute GV bleeding [73][74] and in secondary prophylaxis [75]. The results showed a GV obliteration rate of 70–100%, with no re-bleeding complications, but one event of major bleeding during the procedure was detected. Several groups of authors encourage the use of glue injection and coils in combination (primary prophylaxis, acute GV bleeding, and secondary prophylaxis), believing in their synergistic activity of hemostasis and reducing the risk of re-bleeding and distal embolization [64][67][70][75][76][77][78][79]. The overall GV obliteration rate was 40–100%, with up to a 20% re-bleeding rate. A retrospective trial that compared EUS-guided CYA injection to EUS-guided coil placement showed similar rates of varix obliteration (complete obliteration was more likely to be achieved in the coil group after a single endoscopic session) and re-bleeding rates. It was shown that patients treated with CYA injection had significantly higher adverse events, but the number of sessions needed was fewer in patients receiving coil embolization [67]. Some of the adverse events associated with coil placement (with or without CYA injection) include abdominal pain, fever, minor and major bleeding, coil migration, and extrusion of coils into the gastric lumen [67][73][75]. Severe complications also included pulmonary embolism in up to 25% of cases [64][77]. In addition to synthetic tissue adhesives, such as CYA, some of the biologic tissue adhesives that have been studied for GV obliteration include thrombin (converts fibrinogen to fibrin and promotes clot production) and AGS that is prepared from purified porcine gelatin and can absorb up to 45 times its weight in whole blood. The studies showed that EUS-guided thrombin injection had no procedure-related complications, making it safe to use in this indication [80]. EUS-guided coil placement followed by AGS injection is a well-tolerated procedure, providing positive results in small case series [81][82]. EUS-guided CYA injection with/without coiling has also been used for duodenal varices [83]. Despite the abovementioned results, the specific role of EUS-guided coil/CYA injection in primary prophylaxis of EGV is not clear yet. Based on the available data, the treatment strategy should imply aggressive retreatment of any residual GV seen on follow-up EUS, with the intention of achieving their complete obliteration. It is advocated that EUS-guided coil and CYA injection have the best efficacy in the treatment of GV. Due to the previously mentioned advantages of coil placements, EUS-guided coil insertion has been given a preference over CYA injection. In one single-center study, a retrospective cohort of patients with active/recent bleeding or high-risk GV treated with direct endoscopic injection was compared with a prospective cohort of similar patients treated with EUS-guided fine needle injection (EUS-FNI). It was concluded that EUS-FNI is the preferred treatment strategy, which is substantiated by results showing decreased rates of bleeding in the EUS-guided CYA injection group of patients with active or recently bleeding GV [20][69]. According to retrospective analysis that compared patients who underwent EUS-guided coil injection with patients who underwent a standard EGD injection of CYA for secondary prophylaxis of GV, the EUS group had a significantly lower rate of re-bleeding [76]. In conclusion, EUS does not have an established role in clinical practice to investigate PH yet. The only distinct indication for EUS-guided treatment is the failure of standard EGD in GV bleeding control [4][61]. In the future, EUS might provide an alternative approach to transjugular intrahepatic portosystemic shunts in cases of refractory ascites and refractory variceal bleed, and more studies are needed before its eventual implementation in humans.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11112021
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 392, 1789–1858.
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370.
- Mccarty, T.R.; Bazarbashi, A.N.; Njei, B.; Ryou, M.; Aslanian, H.R.; Muniraj, T. Endoscopic Ultrasound-Guided, Percutaneous, and Transjugular Liver Biopsy: A Comparative Systematic Review and Meta-Analysis. Clin. Endosc. 2020, 53, 583–593.
- Rimbaş, M.; Di Maurizio, L.; Rizzatti, G.; Gasbarrini, A.; Costamagna, G.; Larghi, A. Endoscopic Ultrasound for the Hepatologist: A Comprehensive Review. Semin. Liver Dis. 2018, 38, 145–159.
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403.
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D. American Association for the Study of Liver Dis-eases Liver biopsy. Hepatology 2008, 49, 1017–1044.
- Kalambokis, G.; Manousou, P.; Vibhakorn, S.; Marelli, L.; Cholongitas, E.; Senzolo, M.; Patch, D.; Burroughs, A.K. Transjugular liver biopsy–Indications, adequacy, quality of specimens, and complications–A systematic review. J. Hepatol. 2007, 47, 284–294.
- Myers, R.P.; Fong, A.; Shaheen, A.A.M. Utilization rates, complications and costs of percutaneous liver biopsy: A population-based study including 4275 biopsies. Liver Int. 2008, 28, 705–712.
- Mammen, T.; Keshava, S.N.; Eapen, C.; Raghuram, L.; Moses, V.; Gopi, K.; Babu, N.S.; Ramachandran, J.; Kurien, G. Transjugular Liver Biopsy: A Retrospective Analysis of 601 Cases. J. Vasc. Interv. Radiol. 2008, 19, 351–358.
- West, J.; Card, T.R. Reduced Mortality Rates Following Elective Percutaneous Liver Biopsies. Gastroenterology 2010, 139, 1230–1237.
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.-Z.; Reddy, K.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614–2618.
- Mathew, A. EUS-Guided Routine Liver Biopsy in Selected Patients. Am. J. Gastroenterol. 2007, 102, 2354–2355.
- Gleeson, F.C.; Clayton, A.C.; Zhang, L.; Clain, J.E.; Gores, G.J.; Rajan, E.; Smyrk, T.C.; Topazian, M.D.; Wang, K.K.; Wiersema, M.J.; et al. Adequacy of Endoscopic Ultrasound Core Needle Biopsy Specimen of Nonmalignant Hepatic Parenchymal Disease. Clin. Gastroenterol. Hepatol. 2008, 6, 1437–1440.
- DeWitt, J.; McGreevy, K.; Cummings, O.; Sherman, S.; LeBlanc, J.K.; McHenry, L.; Al-Haddad, M.; Chalasani, N. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest. Endosc. 2009, 69, 535–542.
- Baron, T.; Parekh, P.; Majithia, R.; Diehl, D.L.; Baron, T.H. Endoscopic ultrasound-guided liver biopsy. Endosc. Ultrasound 2015, 4, 85–91.
- Stavropoulos, S.N.; Im, G.; Jlayer, Z.; Harris, M.D.; Pitea, T.C.; Turi, G.K.; Malet, P.F.; Friedel, D.M.; Grendell, J.H. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest. Endosc. 2012, 75, 310–318.
- Vilmann, P.; Krasnik, M.; Larsen, S.S.; Jacobsen, G.K.; Clementsen, P.F. Transesophageal Endoscopic Ultrasound-Guided Fine-Needle Aspiration (EUS-FNA) and Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) Biopsy: A Combined Approach in the Evaluation of Mediastinal Lesions. Endoscopy 2005, 37, 833–839.
- Johnson, K.D.; Laoveeravat, P.; Yee, E.U.; Perisetti, A.; Thandassery, R.B.; Tharian, B. Endoscopic ultrasound guided liver biopsy: Recent evidence. World J. Gastrointest. Endosc. 2020, 12, 83–97.
- Diehl, D.L. Endoscopic Ultrasound–guided Liver Biopsy. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 173–186.
- Mohan, B.P.; Shakhatreh, M.; Garg, R.; Ponnada, S.; Adler, D.G. Efficacy and safety of EUS-guided liver biopsy: A systematic review and meta-analysis. Gastrointest. Endosc. 2019, 89, 238–246.e3.
- Confer, B.D.; Walker, J.T.; Khurana, S.; Unzueta, A.; Khara, H.S.; Johal, A.S.; Diehl, D.L. EUS-guided liver biopsy: The type of needle matters. Gastrointest. Endosc. 2019, 90, 321–322.
- Sey, M.S.L.; Al-Haddad, M.; Imperiale, T.F.; McGreevy, K.; Lin, J.; DeWitt, J.M. EUS-guided liver biopsy for parenchymal disease: A comparison of diagnostic yield between two core biopsy needles. Gastrointest. Endosc. 2016, 83, 347–352.
- Schulman, A.R.; Thompson, C.C.; Odze, R.; Chan, W.W.; Ryou, M. Optimizing EUS-guided liver biopsy sampling: Comprehensive assessment of needle types and tissue acquisition techniques. Gastrointest. Endosc. 2017, 85, 419–426.
- Mok, S.R.S.; Diehl, D.L.; Johal, A.S.; Khara, H.S.; Confer, B.D.; Mudireddy, P.R.; Kovach, A.H.; Diehl, M.M.; Kirchner, H.L.; Chen, Z.-M.E. Endoscopic ultrasound-guided biopsy in chronic liver disease: A randomized comparison of 19-G FNA and 22-G FNB needles. Endosc. Int. Open 2019, 7, E62–E71.
- Obaitan, I.; Al-Haddad, M.A. How Can We Optimize Tools and Techniques for Endoscopic Ultrasound–Guided Liver Biopsy? Clin. Gastroenterol. Hepatol. 2020, 18, 1025–1027.
- Ching-Companioni, R.A.; Diehl, D.L.; Johal, A.S.; Confer, B.D.; Khara, H.S. 19 G aspiration needle versus 19 G core biopsy needle for endoscopic ultrasound-guided liver biopsy: A prospective randomized trial. Endoscopy 2019, 51, 1059–1065.
- Hasan, M.K.; Kadkhodayan, K.; Idrisov, E.; Ali, S.; Rafiq, E.; Shor, D.B.-A.; Abdel-Jalil, A.; Navaneethan, U.; Bang, J.; Varadarajulu, S.; et al. Endoscopic ultrasound-guided liver biopsy using a 22-G fine needle biopsy needle: A prospective study. Endoscopy 2019, 51, 818–824.
- Nieto, J.; Khaleel, H.; Challita, Y.; Jimenez, M.; Baron, T.H.; Walters, L.; Hathaway, K.; Patel, K.; Lankarani, A.; Herman, M.; et al. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest. Endosc. 2018, 87, 469–475.
- Mok, S.R.; Diehl, D.L.; Johal, A.S.; Khara, H.S.; Confer, B.D.; Mudireddy, P.R.; Kirchner, H.L.; Chen, Z.-M.E. A prospective pilot comparison of wet and dry heparinized suction for EUS-guided liver biopsy (with videos). Gastrointest. Endosc. 2018, 88, 919–925.
- Baran, B.; Kale, S.; Patil, P.; Kannadath, B.; Ramireddy, S.; Badillo, R.; DaVee, R.T.; Thosani, N. Endoscopic ultrasound-guided parenchymal liver biopsy: A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 5546–5557.
- Shahidi, N.; Ou, G.; Lam, E.; Enns, R.; Telford, J. When trainees reach competency in performing endoscopic ultrasound: A systematic review. Endosc. Int. Open 2017, 5, E239–E243.
- Chetwood, J.D.; Mudaliar, S.; Staudenmann, D.; Shin, J.-S.; Liu, K.; Majumdar, A.; Kaffes, A.; Strasser, S.; McCaughan, G.W.; Saxena, P. Emerging role of endoscopic ultrasound-guided liver biopsy. Gut 2020, 70, 1600–1601.
- Campos, S.; Poley, J.-W.; Van Driel, L.; Bruno, M.J. The role of EUS in diagnosis and treatment of liver disorders. Endosc. Int. Open 2019, 7, E1262–E1275.
- Suk, K.T. Hepatic venous pressure gradient: Clinical use in chronic liver disease. Clin. Mol. Hepatol. 2014, 20, 6–14.
- Escorsell, À.; Bru, C.; Gilabert, R.; Moitinho, E.; Garcia-Pagan, J.C.; Bosch, J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology 1999, 30, 1393–1397.
- Fujii-Lau, L.; Leise, M.; Kamath, P.; Gleeson, F.; Levy, M. Endoscopic ultrasound-guided portal-systemic pressure gradient measurement. Endoscopy 2014, 46, E654–E656.
- Huang, J.Y.; Samarasena, J.B.; Tsujino, T.; Lee, J.; Hu, K.-Q.; McLaren, C.E.; Chen, W.-P.; Chang, K.J. EUS-guided portal pressure gradient measurement with a simple novel device: A human pilot study. Gastrointest. Endosc. 2017, 85, 996–1001.
- Zhang, W.; Peng, C.; Zhang, S.; Huang, S.; Shen, S.; Xu, G.; Zhang, F.; Xiao, J.; Zhang, M.; Zhuge, Y.; et al. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest. Endosc. 2021, 93, 565–572.
- Bazarbashi, A.N.; Ryou, M. Portal pressure measurement: Have we come full circle? Gastrointest. Endosc. 2021, 93, 573–576.
- Braden, B.; Gupta, V.; Dietrich, C. Therapeutic EUS: New tools, new devices, new applications. Endosc. Ultrasound 2019, 8, 370–381.
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460.
- El-Saadany, M.; Jalil, S.; Irisawa, A.; Shibukawa, G.; Ohira, H.; Bhutani, M.S. EUS for portal hypertension: A comprehensive and critical appraisal of clinical and experimental indications. Endoscopy 2008, 40, 690–696.
- Fung, B.M.; Abadir, A.P.; Eskandari, A.; Levy, M.J.; Tabibian, J.H. Endoscopic ultrasound in chronic liver disease. World J. Hepatol. 2020, 12, 262–276.
- Irisawa, A.; Obara, K.; Sato, Y.; Saito, A.; Takiguchi, F.; Shishido, H.; Sakamoto, H.; Kasukawa, R. EUS analysis of collateral veins inside and outside the esophageal wall in portal hypertension. Gastrointest. Endosc. 1999, 50, 374–380.
- Saraireh, H.A.; Bilal, M.; Singh, S. Role of endoscopic ultrasound in liver disease: Where do we stand in 2017? World J. Hepatol. 2017, 9, 1013–1021.
- Irisawa, A.; Saito, A.; Obara, K.; Shibukawa, G.; Takagi, T.; Shishido, H.; Sakamoto, H.; Sato, Y.; Kasukawa, R. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: Severe periesophageal collateral veins and large perforating veins. Gastrointest. Endosc. 2001, 53, 77–84.
- Mašalaitė, L.; Valantinas, J.; Stanaitis, J. Endoscopic ultrasound findings predict the recurrence of esophageal varices after endoscopic band ligation: A prospective cohort study. Scand. J. Gastroenterol. 2015, 50, 1322–1330.
- Sato, T.; Yamazaki, K.; Toyota, J.; Karino, Y.; Ohmura, T.; Akaike, J. Endoscopic ultrasonographic evaluation of hemodynamics related to variceal relapse in esophageal variceal patients. Hepatol. Res. 2009, 39, 126–133.
- Jeong, S.W.; Kim, H.S.; Kim, S.G.; Yoo, J.-J.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Lee, J.S.; Kim, Y.S.; Kim, B.S. Useful Endoscopic Ultrasonography Parameters and a Predictive Model for the Recurrence of Esophageal Varices and Bleeding after Variceal Ligation. Gut Liver 2017, 11, 843–851.
- Seicean, A. Endoscopic ultrasound in the diagnosis and treatment of upper digestive bleeding: A useful tool. J. Gastrointest. Liver Dis. 2013, 22, 465–469.
- Nagamine, N.; Ueno, N.; Tomiyama, T.; Aizawa, T.; Tano, S.; Wada, S.; Suzuki, T.; Amagai, K.; Ono, K.; Kumakura, Y.; et al. A Pilot Study on Modified Endoscopic Variceal Ligation Using Endoscopic Ultrasonography with Color Doppler Function. Am. J. Gastroenterol. 1998, 93, 150–155.
- De Paulo, G.A.; Ardengh, J.C.; Nakao, F.S.; Ferrari, A.P. Treatment of esophageal varices: A randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest. Endosc. 2006, 63, 396–402.
- Sarin, S.K.; Lahoti, D.; Saxena, S.P.; Murthy, N.S.; Makwana, U.K. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992, 16, 1343–1349.
- Choudhuri, G.; Dhiman, R.K.; Agarwal, D.K. Endosonographic evaluation of the venous anatomy around the gas-tro-esophageal junction in patients with portal hypertension. Hepatogastroenterology 1996, 43, 1250–1255.
- Lee, Y.T.; Chan, F.K.; Ching, J.Y.L.; Lai, C.W.; Leung, V.K.S.; Chung, S.C.S.; Sung, J.J.Y. Diagnosis of Gastroesophageal Varices and Portal Collateral Venous Abnormalities by Endosonography in Cirrhotic Patients. Endoscopy 2002, 34, 391–398.
- Rana, S.S.; Bhasin, D.K.; Singh, K. Duodenal Varix Diagnosed by Endoscopic Ultrasound. Clin. Gastroenterol. Hepatol. 2010, 8, A24.
- Shim, J.-J. Usefulness of Endoscopic Ultrasound in Esophagogastric Varices. Clin. Endosc. 2012, 45, 324–327.
- Thiruvengadam, S.S.; Sedarat, A. The Role of Endoscopic Ultrasound (EUS) in the Management of Gastric Varices. Curr. Gastroenterol. Rep. 2021, 23, 1.
- Schiano, T.D.; Adrain, A.L.; Vega, K.J.; Liu, J.-B.; Black, M.; Miller, L.S. High-resolution endoluminal sonography assessment of the hematocystic spots of esophageal varices. Gastrointest. Endosc. 1999, 49, 424–427.
- Irisawa, A.; Shibukawa, G.; Takagi, T.; Hikichi, T.; Obara, K.; Ohira, H.; Imamura, H. Echo-endoscopic analysis of variceal hemodynamics in patient with isolated gastric varices. Endosc. Ultrasound 2014, 3, 238–244.
- Tang, R.S.; Teoh, A.Y.; Lau, J.Y. EUS-guided cyanoacrylate injection for treatment of endoscopically obscured bleeding gastric varices. Gastrointest. Endosc. 2015, 83, 1032–1033.
- Ma, L.; Tseng, Y.; Luo, T.; Wang, J.; Lian, J.; Tan, Q.; Li, F.; Chen, S. Risk stratification for secondary prophylaxis of gastric varices due to portal hypertension. Dig. Liver Dis. 2019, 51, 1678–1684.
- Lee, Y.T.; Chan, F.K.; Ng, E.K.; Leung, V.K.; Law, K.B.; Yung, M.Y.; Chung, S.; Sung, J.J.Y. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest. Endosc. 2000, 52, 168–174.
- Bhat, Y.M.; Weilert, F.; Fredrick, R.T.; Kane, S.D.; Shah, J.N.; Hamerski, C.M.; Binmoeller, K.F. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: A large U.S. experience over 6 years (with video). Gastrointest. Endosc. 2016, 83, 1164–1172.
- Romero-Castro, R.; Pellicer-Bautista, F.J.; Jimenez-Saenz, M.; Marcos-Sanchez, F.; Caunedo-Alvarez, A.; Ortiz-Moyano, C.; Gomez-Parra, M.; Herrerias-Gutierrez, J.M. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: Results in 5 cases. Gastrointest. Endosc. 2007, 66, 402–407.
- Franco, M.C.; Gomes, G.F.; Nakao, F.S.; De Paulo, G.A.; Jr, A.P.F.; Jr, E.D.L. Efficacy and safety of endoscopic prophylactic treatment with undiluted cyanoacrylate for gastric varices. World J. Gastrointest. Endosc. 2014, 6, 254–259.
- Romero-Castro, R.; Ellrichmann, M.; Ortiz-Moyano, C.; Subtil-Inigo, J.C.; Junquera-Florez, F.; Gornals, J.B.; Repiso-Ortega, A.; Vila-Costas, J.; Marcos-Sanchez, F.; Muñoz-Navas, M.; et al. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: A multicenter study (with videos). Gastrointest. Endosc. 2013, 78, 711–721.
- Gonzalez, J.-M.; Giacino, C.; Pioche, M.; Vanbiervliet, G.; Brardjanian, S.; Ah-Soune, P.; Vitton, V.; Grimaud, J.-C.; Barthet, M. Endoscopic ultrasound-guided vascular therapy: Is it safe and effective? Endoscopy 2012, 44, 539–542.
- Bick, B.L.; Al-Haddad, M.; Liangpunsakul, S.; Ghabril, M.S.; DeWitt, J.M. EUS-guided fine needle injection is superior to direct endoscopic injection of 2-octyl cyanoacrylate for the treatment of gastric variceal bleeding. Surg. Endosc. 2018, 33, 1837–1845.
- Binmoeller, K.F.; Weilert, F.; Shah, J.N.; Kim, J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest. Endosc. 2011, 74, 1019–1025.
- Rose, S.C. Mechanical Devices for Arterial Occlusion and Therapeutic Vascular Occlusion Utilizing Steel Coil Technique: Clinical Applications. Am. J. Roentgenol. 2009, 192, 321–324.
- Romero-Castro, R.; Pellicer-Bautista, F.; Giovannini, M.; Marcos-Sánchez, F.; Caparros-Escudero, C.; Jiménez-Sáenz, M.; Gomez-Parra, M.; Arenzana-Seisdedos, A.; Leria-Yebenes, V.; Herrerias-Gutiérrez, J.M. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy 2010, 42, E35–E36.
- Khoury, T.; Massarwa, M.; Daher, S.; Benson, A.A.; Hazou, W.; Israeli, E.; Jacob, H.; Epstein, J.; Safadi, R. Endoscopic Ultrasound-Guided Angiotherapy for Gastric Varices: A Single Center Experience. Hepatol. Commun. 2018, 3, 207–212.
- Mosquera-Klinger, G.; De La Serna, C.; Bazaga, S.; García-Alonso, J.; Calero-Aguilar, H.; De Benito, M.; Hernández, R.S.-O.; Perez-Miranda, M. Obliteration of Gastric Varices Guided by Eco-Endoscopy with Coils Insertion Coated with Expandable Hidrogel Polymers. Rev. Española De Enferm. Dig. 2020, 113, 352–355.
- Fujii-Lau, L.L.; Law, R.; Song, L.M.W.K.; Gostout, C.J.; Kamath, P.S.; Levy, M.J. Endoscopic ultrasound (EUS)-guided coil injection therapy of esophagogastric and ectopic varices. Surg. Endosc. 2016, 30, 1396–1404.
- Mukkada, R.J.; Antony, R.; Chooracken, M.J.; Francis, J.V.; Chettupuzha, A.P.; Mathew, P.G.; Augustine, P.; Koshy, A. Endoscopic ultrasound-guided coil or glue injection in post-cyanoacrylate gastric variceal re-bleed. Indian J. Gastroenterol. 2018, 37, 153–159.
- Lôbo, M.R.D.A.; Chaves, D.M.; De Moura, D.T.H.; Ribeiro, I.B.; Ikari, E.; De Moura, E.G.H. Safety and efficacy of eus-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: A randomized controlled trial. Arq. De Gastroenterol. 2019, 56, 99–105.
- Kozieł, S.; Pawlak, K.; Błaszczyk, Ł.; Jagielski, M.; Wiechowska-Kozłowska, A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J. Clin. Med. 2019, 8, 1786.
- Kouanda, A.; Binmoeller, K.; Hamerski, C.; Nett, A.; Bernabe, J.; Shah, J.; Bhat, Y.; Watson, R. Safety and efficacy of EUS-guided coil and glue injection for the primary prophylaxis of gastric variceal hemorrhage. Gastrointest. Endosc. 2021, 94, 291–296.
- Frost, J.W.; Hebbar, S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc. Int. Open 2018, 6, E664–E668.
- Bazarbashi, A.N.; Wang, T.J.; Thompson, C.C.; Ryou, M. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable hemostatic gelatin sponge: A novel alternative to cyanoacrylate. Endosc. Int. Open 2020, 8, E221–E227.
- Ge, P.S.; Bazarbashi, A.N.; Thompson, C.C.; Ryou, M. Successful EUS-guided treatment of gastric varices with coil embolization and injection of absorbable gelatin sponge. VideoGIE 2019, 4, 154–156.
- Rana, S.; Bhasin, D.; Chaudhary, V.; Sharma, V.; Sharma, R.; Singh, K. Clinical, endoscopic and endoscopic ultrasound features of duodenal varices: A report of 10 cases. Endosc. Ultrasound 2014, 3, 54–57.
This entry is offline, you can click here to edit this entry!
