Cathodic corrosion is evidenced by the formation of transient complexes of palladium. It is obvious to see a peak of palladium transient by cyclic voltammetry for different amounts of deposited hydrogen expressed as a current during back-diffusion. Therefore, in the part located at the surface of electrolyte, drastic structural changes lead to loss of cohesion and cracking.
The objective of this project is to take into account the mechanical constraints formed by diffusion of hydrogen or isotope and the cathodic corrosion produced by formation of superficial hydride transients, both responsible of destruction of palladium or alloyed cathode. To know the origin of these, it was necessary to discriminating the damaging effects encountered.
- hydrogen isotope
- electrolyzer
- cathode
- diffusion
- palladium alloy
- stress corrosion cracking
- cathodic corrosion
Introduction
Hydrogen is an alternative fuel reducing dependence on fossil fuels and a resource that respects the environment with zero-emission of greenhouse gases. One attractive way of producing high purity hydrogen isotope is electrolysis using watertight palladium diffusion cathode at double face for adsorption, diffusion and desorption in the back side as seen in [1]. In another application concerning nuclear fusion energy systems, tritiated water can be treated by this innovating electrolysis process using the same palladium cathode to produce pure tritium.
The objective of this project is to take into account the mechanical constraints formed by diffusion of hydrogen isotope and the cathodic corrosion produced by formation of superficial hydride transients responsible of destruction of palladium or alloyed cathode. To know the origin of these, it was necessary to understand the damaging effects encountered.
As an example, considering the part of cathode shaped as a finger, in the part emerged above the surface of electrolyte, there is no hydrogen charging in the palladium cathode, it is not the case in the part immersed where electrolysis is loading. Therefore, in the narrow ledge between these two parts, drastic structural changes lead to grain coarsening, loss of cohesion and mechanical constraints. In this case, among the models leading to corrosion by hydrogen and in an alkaline medium, two models can be retained.
Model based on weaker cohesion force
Cathodic corrosion occurs at a sufficiently negative potential to be in presence of superficial absorbed hydrogen or isotope promoting transient hydride palladium formation leading to severe loss of cohesion and weathering of metal [2]. Cathodic corrosion would weaken the superficial structure of palladium, leading to degradation of surface. This type of model is derived from the concept that hydrogen influences breaks in atomic bonds along micro-precipitates and acts on interatomic cohesion force. It can be also called disbonding corrosion, and it results from the hydrogen supersaturation in the inlet face of cathode to lead to the surface decohesion of palladium. Decohesion is accelerated at alkaline pH. As it is based on the weaker cohesion force, it is comparable to the energy model based on surface mechanical failure, except that here it is not a mechanical force but the action of hydrogen absorbed into the palladium cathode in alkaline pH medium. Hydrogen acts as a driver for disbonding. The brittle decohesion mechanism implies that it occurs when the energy of the local stress exceeds that of the intermetallic bonding stress and that the presence of hydrogen absorbed at the surface reduces this cohesion force. This model assumes very high local concentration of absorbed hydrogen in the input face lattice. Considering the face centered cubic lattice du palladium, the interstices are of two types, namely, tetrahedral and octahedral in which interstitial hydrogen atom would have four and six nearest palladium neighbors, respectively (Figure 1). It is expected partial interstitial rearrangement of the atoms in the lattice on the surface from octahedral to tetrahedral sites leading to a morphological change of the surface. The hydrogen traps, privileged sites for supersaturation of this gas, and the cyclic electrolysis loading can promote these changes, so the surface is stripped to leave micro-precipitates. In these regions, transient pseudo-hydrides which are in fact solid solution with high hydrogen content can be formed locally in palladium, and thus hardening and plastic deformation are concerned. This decohesion accompanied by formation of transient hydride can be demonstrated by cyclic voltammetry coupled to electrochemical scanning tunneling microscopy [3]. This process weakens the structure of the metal lattice, leading to degradation of the surface and decohesion of micro-precipitates.
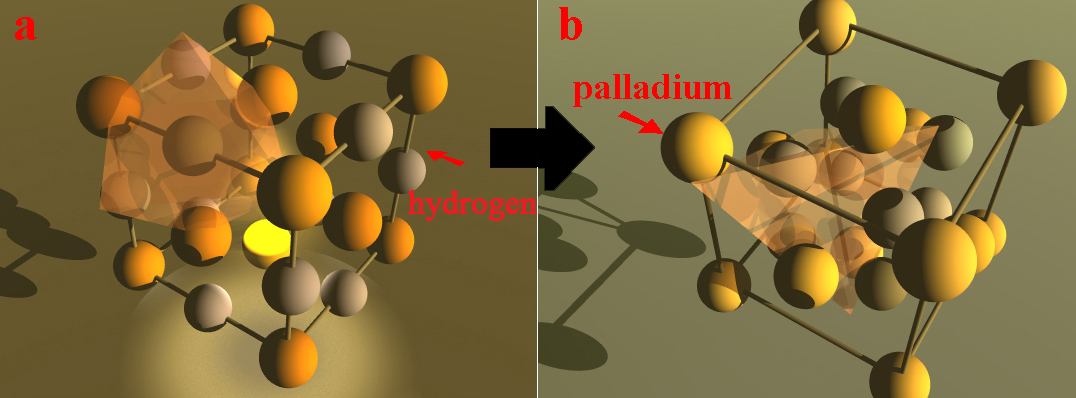
Figure 1. a: octahedral site, b: tetrahedral site for hydrogen.
Model based on hydride formation and brittle phase transformation
Effectively, hydrogen or isotope taking place during diffusion distends the palladium by production of three solid phases not miscible of different size and in three regions of cathode requiring more space for metal, leading to deformation and embrittlement [4]. The presence of these phases modifies the mechanical behavior and induces stress corrosion cracking (SCC) [5]. This mechanism is a source of modification in the lattice, thickening, irreversible process of loss of permeability, and over concentration of proton or hydrogen in the cathode. These drastic structural changes lead to crumpled cathode and mechanical constraints. This destructive effect is more and more progressing limiting the lifetime of electrolyzer. Palladium can be alloyed with silver or yttrium to favorably increase diffusion and reduce these constraints. Using palladium-silver alloy, the cathode has not the sufficient mechanical quality, and it requires porous support that has to contain lattice expansion so that to limit deformation and damage. Support has to possess similar expansion to palladium alloy to ensure good adhesion. Flow in the support and permeation in the palladium have to be equilibrated to avoid over concentration of hydrogen at the interface cathode-support [6].
Stress corrosion cracking is a type of corrosion due to stress in a material in the presence of an aggressive medium. The presence of hydrogen activity and an alkaline medium can drastically increase the corrosivity of the medium and hence play a role in crack propagation. During the corrosion of palladium by cracking, the hydrogen atoms generated by the water reduction reaction cannot recombine to give gaseous dihydrogen. The kinetic of adsorption on palladium is faster than that of the recombination of the hydrogen atoms. These hydrogen atoms adsorb and then diffuse by interstitial diffusion inside the palladium as proton and can accumulate in structural imperfections to form trapped gaseous dihydrogen. These gaseous molecules voluminous at microscopic scale, trapped, cannot diffuse as proton inside the palladium, allowing obtain very high internal pressure in order of 109 Pa and will damage the cathode. This phenomenon of trapping gaseous hydrogen is called "hydrogen embrittlement". The internal pressure of hydrogen contributes to cracking due to the important activity of hydrogen by a fugacity greater than 109 Pa. In these constraint traps, with high hydrogen pressure, the chemical potential decreases, thus favoring a strong hydride precipitation accompanied by a distortion of the lattice. Hydride formed being brittle, a crack begins and spreads to the palladium up to the nonhydrided base to stop. Trapped hydrogen and hydride in the crack tip can be re-formed at this new location by the same process and the sequence of cracking propagation starts again until final rupture [7]. This type of embrittlement is manifested by a degradation of the mechanical characteristics of palladium with plastic deformation. The presence of such quantity of hydrogen favors the formation of brittling phase and stress. In this system interstitial diffusion must be distinguished which is reversible by proton in regard to irreversible trapping. A drop in the characteristic of ductility is observed when the palladium contains irreversibly trapped hydrogen in supersaturation. It is possible to define a stress intensity factor due to the critical hydrogen concentration. This factor will determine when the crack would appear. In the case of circular cracking along the circumference of the glove-shaped diffusion cathode, the stress intensity factor K can be estimated from the the radius of the finger a, the intensity of the stress σ depending on the internal pressure of the trapped hydrogen [8]:
K = 2 π-1 σ√{πa}
Using palladium-yttrium alloy permits more easily to disregard the drawbacks encountered for the palladium and palladium-silver alloy used for the diffusion cathode [9].
Overview
Cathodic corrosion and stress corrosion cracking presented here can intervene separately or in concert in a more or less important network according to their force brought into play since they form in the same range of potential in presence of hydride (Figure 2). In fact, it can be retained there is no stability domain for palladium at alkaline pH medium in presence of hydrogen. Other parameters can also influence cathodic corrosion or stress corrosion cracking. Cathodic corrosion is evidenced by the formation of transient complexes of palladium. It is obvious to see a peak of palladium transient by cyclic voltammetry for different amounts of deposited hydrogen expressed as a current during back-diffusion. These are given in more detail in [10] .
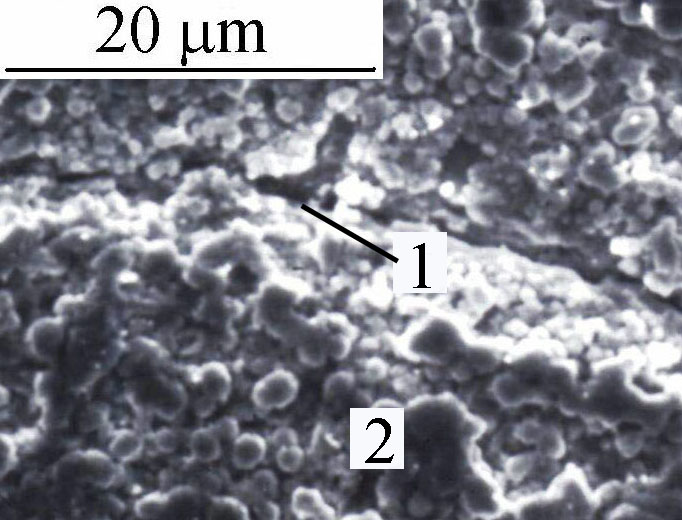
Figure 2. Scanning electron microscopy photograph of palladium cathode showing cracking (1), and loss of cohesion (2) by hydrogen charging.
References
- Bellanger, G . Corrosion Induced by Low-Energy Radionuclides - Modeling of Tritium and Its Radiolytic and Decay Products Formed in Nuclear Installations; Elsevier Science: Amsterdam, 2004; pp. 1-524.
- Yanson, A.I.; Antonov, P.V.; Rodriguez, P.; Koper, M.T.M.; Influence of the electrolyte concentration on the size and shape of platinum nanoparticles synthesized by cathodic corrosion. Electrochim. Acta 2013, 112, 913-918, 10.1016/j.electacta.2013.01.056.
- Sheridan, L.B.; Kim, Y.G.; Perdue, B.R.; Jagannathan, K.; Stickney, J.L.; Robinson, D.B.; Hydrogen adsorption, absorption and desorption at palladium nanofilms formed on Au(111) by electrochemical atomic layer deposition (E-ALD): studies using voltammetry and in situ scanning tunneling microscopy. J. Phys. Chem. C 2013, 117(30), 15728–15740, 10.1021/jp404723a.
- Tosti, S.; Overview of Pd-based membranes for producing pure hydrogen and state of art at ENEA laboratories . Int. J. Hydrogen Energy 2010, 35, 12650-12659, 10.1016/j.ijhydene.2010.07.116.
- Grant, J.L . Palladium, Yttrium, Copper Ternary Alloys for Hydrogen Purification; Ph.D. Thesis, Eds.; University of Birmingham: Birmingham, 2009; pp. 1-98.
- Fletcher, S . Thin Film Palladium-Yttrium Membranes for Hydrogen Separation; Ph.D. Thesis, Eds.; University of Birmingham: Birmingham, 2009; pp. 1-268.
- Coudreuse, L.. Corrosion sous contrainte – phénoménologie et mécanismes; CNRS, Eds.; Les Editions de Physique: Les Ulis, France, 1992; pp. 397-424.
- François, D.. Corrosion sous contrainte – phénoménologie et mécanismes; CNRS, Eds.; Les Editions de Physique: Les Ulis, France , 1992; pp. 211-270.
- Wang, D.; Flanagan, T.B.; Shanahan, K.; Diffusion of H through Pd-Y alloy membranes. J. Membr. Sci. 2016, 499, 452–461, 10.1016/j.memsci.2015.10.020.
- Bellanger Gilbert; Prospecting Stress Formed by Hydrogen or Isotope Diffused in Palladium Alloy Cathode. Materials 2018, 11, 2101, 10.3390/ma11112101.
