Adsorption of pesticides onto natural clay mineral relies on the use of adsorbents with minimal treatment beyond their preparation to provide a narrow size distribution and homoionic form by exchanging the naturally occurring interlamellar cations (in the case of smectites) by some alkaline (Na+ or K+) or alkaline earth (Ca2+or Mg2+) cation. Additional modifications include organophilization, intercalation with metal polycations and pillaring. The adsorption capacity and strength of pesticides onto homoionic, organophilic and intercalated/pillared clay minerals depend on the chemical nature of the pesticide, surface area, and pore volume. Electrostatic interactions, hydrogen and coordinative bonds, surface complexations, and hydrophobic associations are the main interactions between pesticides and clay minerals.
- montmorillonite
- vermiculite
- bentonite
- organoclays
- pesticides
- modified clay minerals
1. Introduction
The so-called Green Revolution dramatically increased agricultural productivity from the middle of the twentieth century to the present [1]. However, this increase relied heavily on chemical fertilizers and a wide range of pesticides, especially herbicides. Consequently, herbicides’ contamination of soils, groundwater, and surface water is a concern of prime importance due to the severe effects of these compounds on humans, animals, and the ecosystem’s equilibrium [2][3][4][5][6].
Adsorption is among the most efficient technologies to prevent or remediate pollution from pesticides because it relies on low-cost materials such as biomaterials, aluminum, and iron oxides, or oxyhydroxides, zeolites, and clay minerals. Clay minerals exhibit properties such as high superficial area, high adsorption capacity, low cost, and ready availability that are valuable to the development of herbicide formulations with controlled releasing of active components [7][8], cleanup of contaminated soils, groundwater protection [9], and water treatment [10][11][12].
Clay minerals have gained interest because they are abundant in nature and are environmentally compatible. Montmorillonite (Mt), like other smectites, and vermiculite (Vt), have permanent negative charges generated by the isomorphic substitutions of Si 4+ by Al 3+ in the tetrahedral sheets and of Al 3+ by Mg 2 + in the octahedrons. Cations such as Na + , Ca 2+ , and Mg 2+ in the interlayer keep the electroneutrality. These permanent negative charges interact with cationic herbicides such as paraquat and diquat [13]. Additionally, these interlayer cations are easily exchangeable, for instance, with organic quaternary ammonium salts to produce hydrophobic organoclays suitable for retaining neutral pesticides [14]. Aluminum, Fe 3+ , Cr 3 + , Ti 4+ , Zr 3+ exchange with the interlayer cations and form polynuclear cationic species under hydrolysis, increasing the affinity towards anionic species [15][16]. Alternatively, the suspension of polycations can be first prepared and then exchanged with the interlayer cation [2]. The exchange of the interlayer cation by a polynuclear hydroxyl metal cation is a modification process named intercalating. Calcinating the intercalated clay minerals produces oxide pillars between the layers, increasing the basal spacing d(001), the specific surface area, and the microporosity, enhancing their adsorption capacity and affinity towards a wide variety of organic molecules, including the pesticides [2][17][18][19].
Excellent reviews recently addressed the interactions between organic compounds and clay minerals, aiming to develop controlled released herbicide formulation and water purification [7][9][20]. A straightforward comparison of adsorbent efficiencies towards various compounds is possible only if parameters such a mass to volume ratio, kinetic, adsorption isotherms, and thermodynamic constants derived from adsorption experiments are presented.
2. Natural Homoionic Clay Minerals as Pesticide Adsorbents
Cation exchange, for instance, is the primary retention mechanism for cationic pesticides such as paraquat, diquat, and difenzoquat on natural and modified clays [13][21][22][23][24][25]. For instance, adsorption isotherms of terbutryn (basic), dicamba (anionic), and paraquat on natural and modified clays from Morocco revealed that the natural clay efficiently adsorbed paraquat from aqueous solutions ( Table 1 ).
| Clay Mineral | Characterization Techniques | Compounds | Adsorbent Concentration (g L−1)/Contact Time (h) | Kinetic Evaluation | Models for Equilibrium Data Treatment | Removal (%) or Adsorption Capacity (Higher Results) | Reference |
|---|---|---|---|---|---|---|---|
| Mt | CEC, SSA, XRD | AT, 2,4-D, paraquat, metsulfuron methyl, glyphosate | 0–8.1/6 | No | Langmuir | Paraquat: 457 μmol g−1, Metsulfuron methyl: 56 μmol g−1, 2,4-D: negligible | [26] |
| Bt, NSC | CEC, SSA, XRD, TG-DTA, organic carbon | Terbutryn, dicamba, paraquat | 10/24 | No | Langmuir, Freundlich | Paraquat: 100% (Bt), 47% (NSC), Dicamba: 30.6% (Bt), 15.2% (NSC), Terbutryn: 11.3% (Bt), 8.29% (NSC) | [21] |
| Bt | CEC, SSA, XRD, XRF, TGA, FTIR | Paraquat | 2.0/24 | No | Langmuir | 111 mg g−1 (403 µmol g−1) | [22] |
| Mt | SEM, TGA, XRD, SSA, FTIR, elemental analysis, zeta potential | Paraquat | -/6 | No | Langmuir | 442 μmol g−1 | [24] |
| Bt, Sepiolite, Illite | CEC, SSA, XRD, TGA, organic carbon | Paraquat | 2.0/24 | No | Freundlich, Langmuir, Dubinin–Radushkevich | 48 μmol g−1 (sepiolite), 212 μmol g−1 (illite), 165 μmol g−1 (Bt) | [27] |
| Mt | SEM, FTIR, SSA, pHzpc | Ametryn | 1.0/6 | Yes | Freundlich, Langmuir, Temkin, | 188.81 mg g−1 (831 µmol g−1) | [28] |
| Bt | CEC, XRD, XRF, SSA, FTIR | Decis (deltametrin) | 20/4 | Yes | Freundlich, Langmuir | 36.39–36.74 mg g−1 (72.0–72.7 µmol g−1) |
[29] |
| Mt, Vt | XRD, SSA, CEC, iron content | AT, DEA, DIA, HAT | 10/24 | No | Freundlich | AT, DIA, HAT: > 99.5% (Mt), DEA: 64-72% (Mt), HAT: > 90% (Vt), AT, DIA: ≈10% (Vt), DEA: negligible (Vt) | [2] |
| Mt | pHzpc, FTIR, Mössbauer, XRD, Na, K, Ca, Mg | Glyphosate | 6.0/24 | No | Freundlich, Langmuir, one/two-sites Sips | 85.64 mg g−1 (507 µmol g−1) at pH 7.0 | [30] |
| Mt | XRD, XPS, SSA, CEC, SEM, chemical composition | Glyphosate | 10/24 | No | Langmuir | 4.0 ± 0.2 μmol m−2 (2.7 × 103) µmol g−1 at pH 4.0 | [31] |
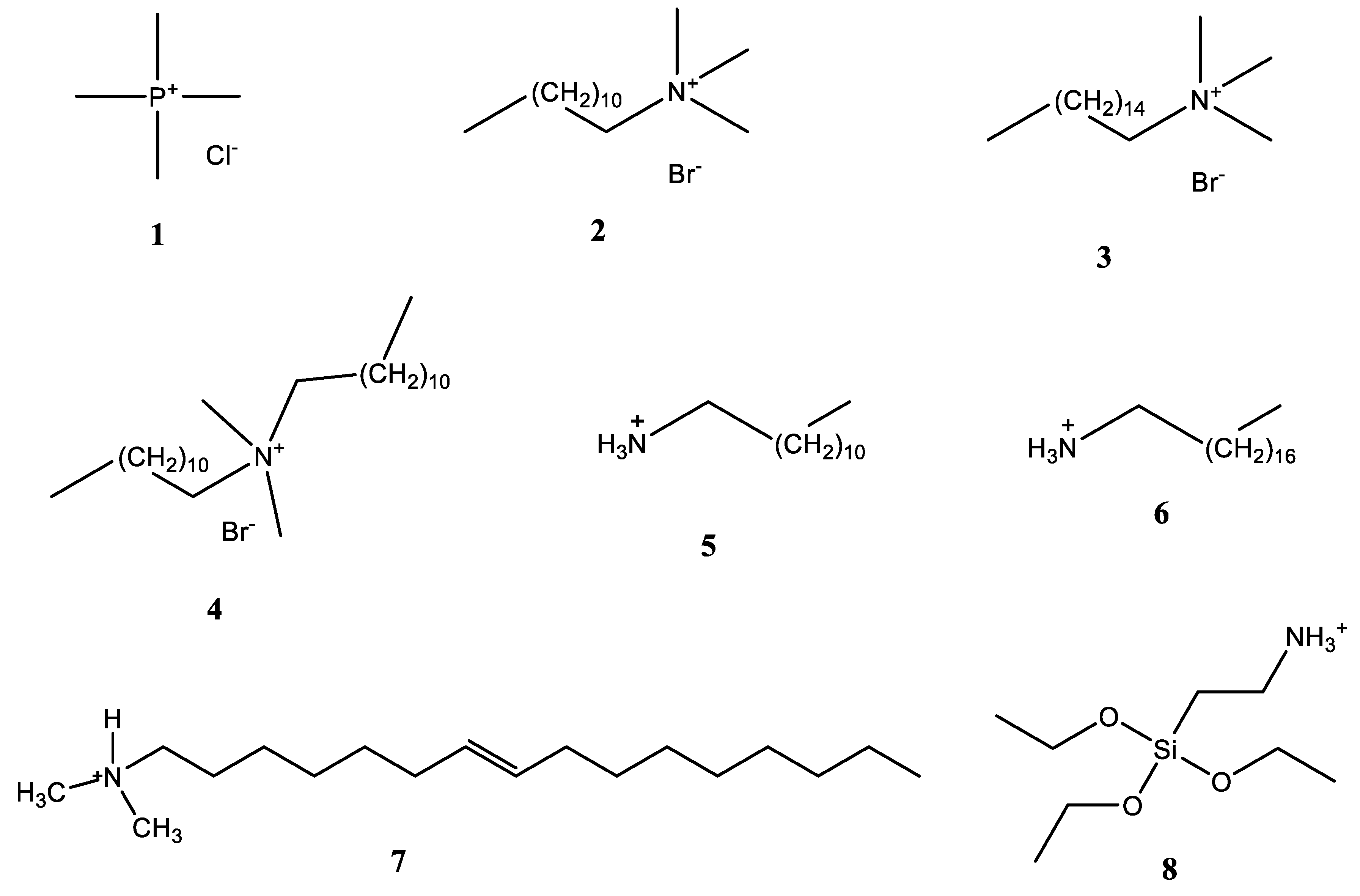
3. Organophilic Clay Minerals
| Clay Mineral | Characterization of the Adsorbents | Compounds | Adsorbent Concentration (g L−1)/Contact Time (h) | Kinetic Evaluation | Models for Equilibrium Data Treatment | Removal (%) or Adsorption Capacities (Higher Results) | Reference |
|---|---|---|---|---|---|---|---|
| Commercial organophilic Bt | SEM-EDX, SSA, TG-DSC, XRF, FTIR | AT, ametryn, 2,4-D, diuron | 5.0/24 | Yes | Langmuir, Freundlich, Temkin | AT: 10.5, ametryn: 111, diuron: 202, 2,4-D: 29 µmol g−1 | [20] |
| Bt and NSC modified with ODTMA, TMA, OTMA | CEC, XRD, SSA, TG-DTA, Organic carbon | Terbutryn, dicamba, paraquat | 10/24 | No | Langmuir, Freundlich | Paraquat: 100% (TMA-Bt), 47% (TMA-NSC), Dicamba: 76.6%, (ODTMA-Bt), 35.5% (ODTMA-NSC), Terbutryn: 95.4% (ODTMA-Bt), 86.5% (ODTMA-NSC) | [21] |
| Mt-Alginate | SEM, TGA, XRD, SSA, FTIR, Zeta Potential, elemental, analysis | Paraquat | -/6 | No | Langmuir | 278 μmol g−1 | [24] |
| Bt, Sepiolite and Illite modified with DDA and NA | CEC, SSA, XRD, TGA, organic carbon | Paraquat | 2.0/24 | No | Freundlich, Langmuir, Dubinin–Radushkevich | 95 μmol g−1 (Illite-DDA), 223 μmol g−1 (Illite-NA) | [27] |
| Kt-TMA, Bt-TMA | CEC, SSA, total organic carbon, elemental analysis | AT, alachlor, trifluralin | 25/548 | No | Freundlich | AT: 69.8% (Bt-TMA), Alachlor: 63.0% (Kt-TMA), Trifluralin: 65.0% (Kt-TMA) | [33] |
| Vt-HDTMA | XRD, SSA, iron content, elemental analysis | Fulvic Acid | 10/24 | No | - | 74 and 98% | [34] |
| Mt-DDTMA, Mt-DDDMA, Mt-HDTMA | XRD, XPS, SSA, FTIR, TGA | AT, imazaquin | 2.5 and 5.0/12 | Yes | Freundlich, Langmuir, | Imazaquin: 35.3 µmol g−1 (Mt-DDDMA), AT: 12.1 µmol g−1 (Mt-HDTMA) | [35] |
| Mt-DDDMA | SEM, SSA, FTIR, XRF, XRD, | Fenitrothion | 0.4/0.25 | No | Freundlich, Langmuir | 68.5 ± 1.2 mg g−1 (247 ± 4 µmol g−1) | [36] |
| Mt-ODA, Mt-DMDA, Mt-ODAAPS | FTIR, XRD, SEM-EDX | chlorpyriphos, p,p′-DDT p,p′-DDE, endosulfan sulphate, α- β-endosulfan, alachlor, metolachlor, fipronil | 10/8 | Yes | Freundlich | (In µmol g−1) p,p′-DDT: 1.47, p,p′-DDE: 1.19, Chlorpyriphos: 1.0, α-endosulfan: 0.84, β-endosulfan: 0.698, endosulfan sulphate: 0.61, fipronil: 0.62, alachlor: 0.70, metolachlor: 0.67 | [37] |
| Mt-carboxy methyl cellulose-DMDA | XRD, SEM-EDX, FTIR | AT, imidacloprid, thiamethoxam | 10/4 | No | Freundlich, Langmuir | Imidacloprid: 8.82, thiamethoxam: 5.71, AT: 6.63 µmol g−1 | [38] |
4. Intercalated and Pillared Clay Minerals
| Modified Clay Mineral | Characterization of the Adsorbents | Compounds | Adsorbent Concentration (g L−1)/Contact Time (h) | Kinetic Evaluations | Model for Equilibrium Data Treatment | Removal (%) or Adsorption Capacity (Higher Results) | Reference |
|---|---|---|---|---|---|---|---|
| Pillared Mt-Fe | XRD, SSA | AT | 10/24 | No | Freundlich | 62.8-99.1% | [17] |
| Intercalated Mt-Fe, Vt-Fe | XRD, SSA, CEC, iron content | AT, DEA, DIA, HAT | 10/24 | No | Freundlich | AT, DEA, DIA, HAT: >94% (Mt-Fe), AT: 3375% (Fe-Vt) | [2] |
| Pillared Bt-Al13 | XRD, CEC, chemical composition | Thiabendazole | 0.6–2.5/24 | No | Freundlich, Langmuir | 141 µmol g−1 (aged 12 h at 60 °C) 318 µmol g−1 (aged 12 h at 25 °C) |
[41] |
| Intercalated and pillared Bt-Al13, Bt-Zr | XRD, CEC, SSA, Chemical composition | AT, 3-CA, 3-CP | 20/overnight | No | Freundlich, Langmuir | AT: 92–100%, 67.1 μmol g−1 (Bt-Al) and 117.6 μmol g−1 (Bt-Zr) 3-CA: 14–100%, 3-CP: 10–30% |
[44] |
| Intercalated Mt-Al13, Mt-Fe, Mt-Ti, modified with CTAB | XRD, SSA, DTA, TGA, CEC, FTIR, surface acidity, Zeta- potential | Diuron, DCPMU, DCPU, DCA | 0.05–0.5/24 | No | Freundlich | Diuron: 15.7, DCPMU: 14.0, DCPU: 6.79, DCA: 6.65 µmol g−1- measured at pH 3.1 and 0.5 g L−1 dispersion | [47] |
| Pillared Mt-Fe | XRD, TG-DTA, SSA, SEM, FTIR, elemental analysis, Mössbauer, Zeta-potential | Picloram | 16/48 | No | Freundlich, Langmuir | 380 μmol g−1 at pH 3.0 | [49] |
| Pillared Mt-Fe-Al13 modified with cyclodextrins | FTIR, XRD, XRF, SSA | Imazaquin | 1.6/24 | No | - | ≈65 μmol g−1 | [50] |
| Pillared Mt-Fe-Al13 modified with cyclodextrins | XRD, SSA, FTIR, SEM-EDX | Picloram | 1.6/24 | No | Freundlich, Langmuir | 380 μmol g−1 | [51] |
| Pillared Bt-Al30 | SEM, SSA, XRD | heptachlor epoxide, dieldrin, endrin | 1.0/5 | Yes | Freundlich, Langmuir | Heptachlor epoxide: 0.62, Dieldrin: 0.63, Endrin: 0.62 µmol g−1 | [62] |
5. Concluding Remarks
This entry is adapted from the peer-reviewed paper 10.3390/min11111282
References
- Pimentel, D. Green revolution agriculture and chemical hazards. Sci. Total Environ. 1996, 188, S86–S98.
- Abate, G.; Masini, J.C. Adsorption of atrazine, hydroxyatrazine, deethylatrazine, and deisopropylatrazine onto Fe(III) polyhydroxy cations intercalated vermiculite and montmorillonite. J. Agric. Food Chem. 2005, 53, 1612–1619.
- Horak, I.; Horn, S.; Pieters, R. Agrochemicals in freshwater systems and their potential as endocrine disrupting chemicals: A South African context. Environ. Pollut. 2021, 268, 115718.
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483.
- Chow, R.; Scheidegger, R.; Doppler, T.; Dietzel, A.; Fenicia, F.; Stamm, C. A review of long-term pesticide monitoring studies to assess surface water quality trends. Water Res. X 2020, 9, 100064.
- Rigobello-Masini, M.; Pereira, E.A.O.; Abate, G.; Masini, J.C. Solid-Phase Extraction of Glyphosate in the Analyses of Environmental, Plant, and Food Samples. Chromatographia 2019, 82, 1121–1138.
- Undabeytia, T.; Shuali, U.; Nir, S.; Rubin, B. Applications of chemically modified clay minerals and clays to water purification and slow release formulations of herbicides. Minerals 2021, 11, 9.
- Natarelli, C.V.L.; Claro, P.I.C.; Miranda, K.W.E.; Ferreira, G.M.D.; de Oliveira, J.E.; Marconcini, J.M. 2,4-Dichlorophenoxyacetic acid adsorption on montmorillonite organoclay for controlled release applications. SN Appl. Sci. 2019, 1, 1–13.
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719.
- Lelario, F.; Gardi, I.; Mishael, Y.; Dolev, N.; Undabeytia, T.; Nir, S.; Scrano, L.; Bufo, S.A. Pairing micropollutants and clay-composite sorbents for efficient water treatment: Filtration and modeling at a pilot scale. Appl. Clay Sci. 2017, 137, 225–232.
- Shabeer, T.P.A.; Saha, A.; Gajbhiye, V.T.; Gupta, S.; Manjaiah, K.M.; Varghese, E. Simultaneous removal of multiple pesticides from water: Effect of organically modified clays as coagulant aid and adsorbent in coagulation-flocculation process. Environ. Technol. 2014, 35, 2619–2627.
- Amari, A.; Alzahrani, F.M.; Katubi, K.M.; Alsaiari, N.S.; Tahoon, M.A.; Rebah, F. Ben Clay-polymer nanocomposites: Preparations and utilization for pollutants removal. Materials 2021, 14, 1365.
- Infante, C.M.C.; Masini, J.C. Development of a spectrophotometric sequential injection methodology for online monitoring of the adsorption of paraquat on clay mineral and soil. Spectrosc. Lett. 2007, 40, 3–14.
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chim. 2019, 22, 132–141.
- Mdlalose, L.; Balogun, M.; Setshedi, K.; Chimuka, L.; Chetty, A. Adsorption of phosphates using transition metals-modified bentonite clay. Sep. Sci. Technol. 2019, 54, 2397–2408.
- Almasri, D.A.; Rhadfi, T.; Atieh, M.A.; McKay, G.; Ahzi, S. High performance hydroxyiron modified montmorillonite nanoclay adsorbent for arsenite removal. Chem. Eng. J. 2018, 335, 1–12.
- Abate, G.; Masini, J.C. Influence of thermal treatment applied to Fe(III) polyhydroxy cation intercalated vermiculite on the adsorption of atrazine. J. Agric. Food Chem. 2007, 55, 3555–3560.
- Khalaf, H.; Bouras, O.; Perrichon, V. Synthesis and characterization of Al-pillared and cationic surfactant modified Al-pillared Algerian bentonite. Microporous Mater. 1997, 8, 141–150.
- Lenoble, V.; Bouras, O.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenic adsorption onto pillared clays and iron oxides. J. Colloid Interface Sci. 2002, 255, 52–58.
- Manzotti, F.; dos Santos, O.A.A. Evaluation of removal and adsorption of different herbicides on commercial organophilic clay. Chem. Eng. Commun. 2019, 206, 1526–1543.
- Azejjel, H.; del Hoyo, C.; Draoui, K.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Natural and modified clays from Morocco as sorbents of ionizable herbicides in aqueous medium. Desalination 2009, 249, 1151–1158.
- Ait Sidhoum, D.; Socías-Viciana, M.M.; Ureña-Amate, M.D.; Derdour, A.; González-Pradas, E.; Debbagh-Boutarbouch, N. Removal of paraquat from water by an Algerian bentonite. Appl. Clay Sci. 2013, 83–84, 441–448.
- Pateiro-Moure, M.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J. Quaternary herbicides retention by the amendment of acid soils with a bentonite-based waste from wineries. J. Hazard. Mater. 2009, 164, 769–775.
- Etcheverry, M.; Cappa, V.; Trelles, J.; Zanini, G. Montmorillonite-alginate beads: Natural mineral and biopolymers based sorbent of paraquat herbicides. J. Environ. Chem. Eng. 2017, 5, 5868–5875.
- Wang, M.; Orr, A.A.; He, S.; Dalaijamts, C.; Chiu, W.A.; Tamamis, P.; Phillips, T.D. Montmorillonites Can Tightly Bind Glyphosate and Paraquat Reducing Toxin Exposures and Toxicity. ACS Omega 2019, 4, 17702–17713.
- Otalvaro, J.O.; Brigante, M. Interaction of pesticides with natural and synthetic solids. Evaluation in dynamic and equilibrium conditions. Environ. Sci. Pollut. Res. 2018, 25, 6707–6719.
- Seki, Y.; Yurdakoç, K. Paraquat adsorption onto clays and organoclays from aqueous solution. J. Colloid Interface Sci. 2005, 287, 1–5.
- Shattar, S.F.A.; Zakaria, N.A.; Foo, K.Y. Utilization of montmorillonite as a refining solution for the treatment of ametryn, a second generation of pesticide. J. Environ. Chem. Eng. 2017, 5, 3235–3242.
- Bouazza, F.; Benguella, B.; Soussi, S. Elimination of pesticides by natural and modified clays. Can. J. Chem. 2018, 96, 975–983.
- Pereira, R.C.; da Costa, A.C.S.; Ivashita, F.F.; Paesano, A.; Zaia, D.A.M. Interaction between glyphosate and montmorillonite in the presence of artificial seawater. Heliyon 2020, 6, e03532.
- Khoury, G.A.; Gehris, T.C.; Tribe, L.; Torres Sánchez, R.M.; dos Santos Afonso, M. Glyphosate adsorption on montmorillonite: An experimental and theoretical study of surface complexes. Appl. Clay Sci. 2010, 50, 167–175.
- Shattar, S.F.A.; Zakaria, N.A.; Foo, K.Y. Feasibility of montmorillonite-assisted adsorption process for the effective treatment of organo-pesticides. Desalin. Water Treat. 2016, 57, 13645–13677.
- Leovac, A.; Vasyukova, E.; Ivančev-Tumbas, I.; Uhl, W.; Kragulj, M.; Tričković, J.; Kerkez, D.; Dalmacija, B. Sorption of atrazine, alachlor and trifluralin from water onto different geosorbents. RSC Adv. 2015, 5, 8122–8133.
- Abate, G.; dos Santos, L.B.O.; Colombo, S.M.; Masini, J.C. Removal of fulvic acid from aqueous media by adsorption onto modified vermiculite. Appl. Clay Sci. 2006, 32, 261–270.
- Park, Y.; Sun, Z.; Ayoko, G.A.; Frost, R.L. Removal of herbicides from aqueous solutions by modified forms of montmorillonite. J. Colloid Interface Sci. 2014, 415, 127–132.
- Oiwa, M.; Yamaguchi, K.; Hayashi, H.; Saitoh, T. Rapid sorption of fenitrothion on didodecyldimethylammonium bromide-montmorillonite organoclay followed by the degradation into less toxic 3-methyl-4-nitrophenolate. J. Environ. Chem. Eng. 2020, 8, 104000.
- Saha, A.; Ahammed, S.T.; Gajbhiye, V.T.; Gupta, S.; Kumar, R. Removal of mixed pesticides from aqueous solutions using organoclays: Evaluation of equilibrium and kinetic model. Bull. Environ. Contam. Toxicol. 2013, 91, 111–116.
- Narayanan, N.; Gupta, S.; Gajbhiye, V.T.; Manjaiah, K.M. Optimization of isotherm models for pesticide sorption on biopolymer-nanoclay composite by error analysis. Chemosphere 2017, 173, 502–511.
- Najafi, H.; Farajfaed, S.; Zolgharnian, S.; Mosavi Mirak, S.H.; Asasian-Kolur, N.; Sharifian, S. A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf. Environ. Prot. 2021, 147, 8–36.
- Galeano, L.A.; Vicente, M.Á.; Gil, A. Catalytic degradation of organic pollutants in aqueous streams by mixed Al/M-pillared clays (M = Fe, Cu, Mn). Catal. Rev. Sci. Eng. 2014, 56, 239–287.
- Roca Jalil, M.E.; Vieira, R.S.; Azevedo, D.; Baschini, M.; Sapag, K. Improvement in the adsorption of thiabendazole by using aluminum pillared clays. Appl. Clay Sci. 2013, 71, 55–63.
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770.
- Khankhasaeva, S.T.; Badmaeva, S.V. Removal of p-aminobenzenesulfanilamide from water solutions by catalytic photo-oxidation over Fe-pillared clay. Water Res. 2020, 185, 116212.
- Matthes, W.; Kahr, G. Sorption of organic compounds by Al and Zr-hydroxy intercalated and pillared bentonite. Clays Clay Miner. 2000, 48, 593–602.
- Herney-Ramirez, J.; Vicente, M.A.; Madeira, L.M. Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review. Appl. Catal. B Environ. 2010, 98, 10–26.
- Cardona, Y.; Korili, S.A.; Gil, A. Understanding the formation of Al13 and Al30 polycations to the development of microporous materials based on Al13-and Al30-PILC montmorillonites: A review. Appl. Clay Sci. 2021, 203, 105996.
- Bouras, O.; Bollinger, J.C.; Baudu, M.; Khalaf, H. Adsorption of diuron and its degradation products from aqueous solution by surfactant-modified pillared clays. Appl. Clay Sci. 2007, 37, 240–250.
- Li, J.; Li, Y.; Lu, J. Adsorption of herbicides 2,4-D and acetochlor on inorganic-organic bentonites. Appl. Clay Sci. 2009, 46, 314–318.
- Marco-Brown, J.L.; Barbosa-Lema, C.M.; Torres Sánchez, R.M.; Mercader, R.C.; dos Santos Afonso, M. Adsorption of picloram herbicide on iron oxide pillared montmorillonite. Appl. Clay Sci. 2012, 58, 25–33.
- Undabeytia, T.; Galán-Jiménez, M.C.; Gómez-Pantoja, E.; Vázquez, J.; Casal, B.; Bergaya, F.; Morillo, E. Fe-pillared clay mineral-based formulations of imazaquin for reduced leaching in soil. Appl. Clay Sci. 2013, 80–81, 382–389.
- Marco-Brown, J.L.; Undabeytia, T.; Torres Sánchez, R.M.; dos Santos Afonso, M. Slow-release formulations of the herbicide picloram by using Fe–Al pillared montmorillonite. Environ. Sci. Pollut. Res. 2017, 24, 10410–10420.
- Vercellone, S.Z.; Sham, E.; Torres, E.M.F. Measure of Zeta Potential of Titanium Pillared Clays. Proc. Mater. Sci. 2015, 8, 599–607.
- Abdennouri, M.; Baâlala, M.; Galadi, A.; El Makhfouk, M.; Bensitel, M.; Nohair, K.; Sadiq, M.; Boussaoud, A.; Barka, N. Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem. 2016, 9, S313–S318.
- Gil, A.; Assis, F.C.C.; Albeniz, S.; Korili, S.A. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040.
- Cabrera-Lafaurie, W.A.; Román, F.R.; Hernández-Maldonado, A.J. Transition metal modified and partially calcined inorganic-organic pillared clays for the adsorption of salicylic acid, clofibric acid, carbamazepine, and caffeine from water. J. Colloid Interface Sci. 2012, 386, 381–391.
- Yan, F.; Spyrou, K.; Thomou, E.; Kumar, S.; Cao, H.; Stuart, M.C.A.; Pei, Y.; Gournis, D.; Rudolf, P. Smectite clay pillared with copper complexed polyhedral oligosilsesquioxane for adsorption of chloridazon and its metabolites. Environ. Sci. Nano 2020, 7, 424–436.
- Zielke, R.C.; Pinnavaia, T.J. Modified clays for the adsorption of environmental toxicants: Binding of chlorophenols to pillared, delaminated, and hydroxy-interlayered smectites. Clays Clay Miner. 1988, 36, 403–408.
- Konstantinou, I.K.; Albanis, T.A.; Petrakis, D.E.; Pomonis, P.J. Removal of herbicides from aqueous solutions by adsorption on Al- pillared clays, Fe-Al pillared clays and mesoporous alumina aluminum phosphates. Water Res. 2000, 34, 3123–3136.
- Roca Jalil, M.E.; Baschini, M.; Rodríguez-Castellón, E.; Infantes-Molina, A.; Sapag, K. Effect of the Al/clay ratio on the thiabendazol removal by aluminum pillared clays. Appl. Clay Sci. 2014, 87, 245–253.
- Cardona, Y.; Korili, S.A.; Gil, A. A nonconventional aluminum source in the production of alumina-pillared clays for the removal of organic pollutants by adsorption. Chem. Eng. J. 2021, 425, 130708.
- Zhu, H.Y.; Li, J.Y.; Zhao, J.C.; Churchman, G.J. Photocatalysts prepared from layered clays and titanium hydrate for degradation of organic pollutants in water. Appl. Clay Sci. 2005, 28, 79–88.
- Farghali, R.A.; Basiony, M.S.; Gaber, S.E.; Ibrahim, H.; Elshehy, E.A. Adsorption of organochlorine pesticides on modified porous Al30/bentonite: Kinetic and thermodynamic studies. Arab. J. Chem. 2020, 13, 6730–6740.
