Dear author, the following contents are excerpts from your papers. They are editable.
(Due to the lack of relevant professional knowledge, our editors cannot complete a complete entry by summarizing your paper, so if you are interested in this work. you may need to write some contents by yourself. A good entry will better present your ideas, research and results to other scholars. Readers will also be able to access your paper directly through entries.)
The terminal portions of human chromosomes are capped by nucleoprotein structures called telomeres [
1,
2,
3,
4]. Telomeres maintain genomic stability in normal somatic cells and protect against chromosomal degradation [
5,
6]. Telomeres are progressively shortened by each round of cell division, and, after approximately 50 divisions, they reach a critically short length that induces DNA damage responses leading to cellular senescence or apoptosis [
7,
8,
9]. Telomerase is an enzymatic ribonucleoprotein complex that maintains telomere length by adding single-stranded TTAGGG stretches to the 3′ end of the chromosome [
6,
8,
10,
11,
12]. Human telomerase is composed of two subunits, a reverse transcriptase component called human telomerase reverse transcriptase (hTERT) and a template RNA component called human telomerase RNA gene (hTERC) [
11,
12,
13]. These two subunits adopt a well-defined tertiary structure in the catalytic core of the telomerase complex and exhibit limited protein-RNA interaction [
12]. Telomerase activity is relatively quiescent in most normal tissue; however, studies have found it to be significantly increased in up to 90% of malignancies [
8,
14,
15,
16]. The activation of telomerase allows cells to circumvent senescence and continue to divide while accumulating oncogenic mutations that can ultimately lead to cancer [
17,
18]. Cancer therapeutics specifically targeting telomerase-positive cells have garnered interest as a possible alternative to traditional chemotherapy and radiotherapy methods, which are toxic to both cancerous and normal cells [
8,
15]. The use of oligonucleotides and microRNAs (miRNAs) that inhibit the function of hTERC and hTERT is a further specified subclass of telomerase-centered therapies that has shown promise in treatment for a number of human malignancies [
19,
20,
21,
22]. This comprehensive review will elaborate on the current state and underlying concepts of cancer therapeutics aimed at reducing telomerase activity by targeting telomerase subunits with oligonucleotide-based approaches.
2. Oligonucleotides
Oligonucleotides are molecules composed of short segments of DNA or RNA that have been used for a variety of purposes in the fields of medicine and biomedical research. Because of their ability to bind to complementary sequences of DNA or RNA, oligonucleotides have been employed as simple nucleic acid probes using a number of basic science laboratory techniques, such as polymerase chain reaction (PCR) and DNA sequencing, as well as in the specific targeting and regulation of the expression and function of a wide range of proteins [
71,
72]. Due to their diverse functionality, oligonucleotides have been at the center of many research studies in numerous disease models [
73]. They have been particularly useful in studying cancer biology, as their ability to bind and regulate specific nucleotide sequences allows for the study of both the etiology and treatment of many different cancers [
15,
19,
21]. Specific oligonucleotide molecules such as miRNAs and AS-ODNs, including T-oligos, have been used to target distinct complementary DNA and RNA sequences in order to modulate both aberrant protein levels and enzyme activities implicated in various cancers [
8,
15,
74]. 6-Thio-2′-Deoxyguanosine (6-Thio-dG) is an effective nucleoside analog and telomerase substrate which brings about several anti-cancer effects through incorporation into DNA [
8,
15].
Much progress has been made in the use of oligonucleotides to target molecular events leading to the upregulation of telomerase [
23,
75]. As previously mentioned, hTERT is the catalytic component of telomerase that is essential for the replication of chromosomal telomeres, a process that confers cells with perpetual replicative potential [
23,
75,
76]. Oligonucleotides can be tailored to target hTERT at specific steps in its synthesis and activity, including at the hTERT promoter, hTERT mRNA, or in its protein form [
15,
77]. In order for hTERT to successfully perform its catalytic function, it must be able to properly interact with telomere-related proteins in the shelterin complex and bind to the telomeric nucleotide sequence using hTERC. Regulation of the hTERT protein itself has been accomplished through oligonucleotide-based interference of hTERT at a number of different points [
8,
15].
AS-ODNs have shown promise in both preclinical and clinical trials as a potential treatment for various cancers. A number of preclinical studies used AS-ODNs to target the hTERT initiator sequence, pre-mRNA, or transcribed mRNA, which significantly decreased hTERT activity and increased growth inhibition in human hepatoma, prostate adenocarcinoma, bladder cancer, and hepatic lymphoma [
24,
78,
79,
80]. In particular, Folini et al. found that antisense oligonucleotide-mediated inhibition of hTERT, but not hTERC, inhibited cell growth and induced apoptosis in human prostate cancer cells, implying that telomerase can retain its role in telomere replication even in the absence of a properly functioning RNA template. Another study found that the simultaneous in vitro targeting of both hTERT and hTERC with AS-ODNS resulted in the synergistic inhibition of growth in human colon cancer cells [
81]. The results of these early preclinical studies showed that hTERT could be successfully targeted at a number of regulatory points involved in its cellular production [
81]. They also demonstrated the wide applicability of specifically engineered oligonucleotides to target cells with abnormal hTERT activity [
81]. Additionally, various preclinical and clinical studies have revealed the synergistic anti-cancer effects of AS-ODNs with other cancer drugs on the inhibition of abnormal cancer cell growth [
82,
83,
84].
Although AS-ODNs can be used to target the production of hTERT, one of the best-studied AS-ODNs GRN163L (Imetelstat) acts as a competitive inhibitor of telomerase activity. GRN163L is a 13-merthiophosphoramidate deoxyribo-oligonucleotide that is a complement and antagonist to the hTERT-associated hTERC RNA template sequence. By binding to hTERC, Imetelstat prevents hTERT-mediated telomere elongation, leading to progressive telomere attrition and eventual cell death [
8]. Imetelstat preferentially targets cancer cells due to their intrinsically high level of telomerase activity and has been tested in a number of preclinical and clinical trials conducted to assess its effect on different types of cancers, with varying levels of success [
82,
84,
85].
In preclinical studies using both in vitro human cells and in vivo murine models, Imetelstat has shown great promise in its ability to decrease the growth of many cancers, as well as downregulate hTERT activity. Imetelstat alone significantly decreased telomere length through hTERT inhibition and increased cell death of human myeloma and pancreatic cancer cells in an in vitro model [
63,
86]. Xenograft murine models showed similar results for both myeloma and malignant rhabdoid tumor cells, demonstrating telomere shortening, decreased growth, and increased cell death [
86,
87]. Additionally, Imetelstat has exhibited anti-metastatic properties in mouse models of lung cancer [
88,
89]. Imetelstat has been tested in numerous preclinical trials involving other types of cancers, both by itself and in combination with other anti-cancer therapeutics, with similar patterns of inhibition of telomerase and decreasing cell viability being observed [
15,
28]. Despite the apparent effectiveness of Imetelstat in preclinical studies, many preclinical trials using it have severe limitations which prevent its applicability to human treatments. Some of these issues include significant differences observed between in vitro and in vivo models for human cells as well as apparent variations between telomere structure and maintenance in mice compared to humans [
15].
Despite its success in preclinical trials, Imetelstat has not had as much success in clinical trials due to its severe hematological side effects, including thrombocytopenia and myelosuppression, and as a result has not been approved by the U.S. Food and Drug Administration (FDA) [
1]. It has been involved in numerous Phase I/Phase II clinical trials that have been completed as well as some Phase II/Phase III trials that are still ongoing [
15,
28,
90]. These studies have shown mixed results, with some reporting no significant increase in long-term patient survival and others reporting no significant reduction in tumor size despite a 95% reduction in telomerase activity [
91,
92].
Preclinical and clinical trials have tested the drug both alone and in combination with other anti-cancer drugs in the treatment of various types of cancers [
1]. The most promising results with Imetelstat have been obtained when it is used alongside other chemotherapeutic agents, such as 3-aminobenzamide (3AB) and trastuzumab, or conventional radiation therapy, possibly through the increased sensitization of cancer cells to these other treatments [
15,
93,
94]. Future studies on Imetelstat should investigate the synergistic targeting of hTERT by Imetelstat in combination with other drugs, such as small molecule inhibitors, that target other sources of cancer aggression while simultaneously minimizing unfavorable toxicity. Both preclinical and clinical trials are currently ongoing in these areas [
15,
28].
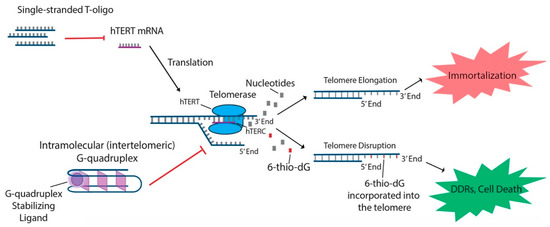
In addition to the aforementioned oligonucleotides, both T-oligos and 6-thio-dG have generated interest due to their potential to regulate hTERT and possible use in treating various cancers. T-oligos are oligonucleotides with a sequence homologous to the 3′ telomere overhang. Their anti-cancer effects are proposed to occur by activation of DNA damage responses (DDRs) and subsequent cell death, either by mimicking damaged DNA or by triggering the dissociation of critical shelterin proteins from telomeres [
15,
27]. T11 is a specific 11-base T-oligo that has been shown to demonstrate anti-cancer properties in a number of different cancers [
8,
15,
27,
28]. T11 is proposed to function through its resemblance to the telomeric overhang DNA sequence and displays promise because it has no effect on normal cells () [
27]. Administration of T11 also had no detectable toxic effects in mice [
28,
95,
96,
97]. In preclinical studies, T-oligo was shown to inhibit mRNA expression of hTERT by 50% in a melanoma cell model [
98]. Additionally, concurrent administration of T-oligo with vemurafenib in V600E-positive melanoma cells demonstrated an additive anti-cancer effect, implicating the potential benefit of T-oligo administration with chemotherapeutics in current use [
98,
99]. 6-thio-dG, a nucleoside analog, has been shown to possess anti-cancer properties by being incorporated into telomeric DNA, leading to the uncapping of telomeric DNA and dissociation of the shelterin complex (). This results in telomere dysfunction-induced foci (TIFs) and subsequent cellular senescence and apoptosis [
82]. Furthermore, this effect has been shown to be partly dependent on the activity of hTERT, as TIFs were not seen in cells lacking hTERT activity [
82]. Future studies should focus on the specific molecular interactions of 6-thio-dG and hTERT in order to better establish the effect of 6-thio-dG on telomerase activity.
Figure 2. T-oligo, 6-thio-dG, and G-quadruplex stabilizing ligands like telomestatin function to prevent telomere elongation through unique processes related to inhibition of the telomerase subunits. T-oligo has been shown to inhibit expression of hTERT mRNA in melanoma cells with activated JNK signaling. 6-thio-dG incorporates into telomeric DNA and stimulates TIFs in cells that exhibit hTERT activity. G-quadruplex stabilizing ligands prevent hTERC from binding to the single-stranded telomere overhang.