Tendon and ligament injury poses an increasingly large burden to society. With surgical repair and grafting susceptible to high failure rates, tissue engineering provides novel avenues for treatment.
- mesenchymal stem cells
- extracellular vesicles
- tendon
- ligament
- biomechanics
- macrophage
1. Introduction
Tendon and ligament injuries make up about 50% of all musculoskeletal injuries and cost $30 billion a year to manage [1]. These can be due to underlying tendon diseases, such as inflammatory or degenerative changes seen in tendinopathies, or due to acute traumatic injury. Tendons generally have limited vascularisation when compared to muscle, especially at the tendon–bone interface. There is reduced angiogenesis at these sites due to inhibitory factors such as endostatin, secreted from neighbouring cells [2]. The blood supply to tendons generally comes from three sources: the myotendinous junction, the osteotendinous junction, and the tendon sheath. Ligaments, on the other hand, have blood supply from the synovium as well as from the surrounding soft tissue. The healing response in ligaments can be divided into the hemorrhagic, inflammatory, reparative, and remodelling phases. The final phase can take up to months or years to complete, and is subject to a number of external factors, with minimal exercise, smoking, and high cholesterol associated with poorer outcomes [3]. These injuries can be severely debilitating, whether in athletes trying to return to their high level of sporting ability, or patients returning to independent living. Furthermore, a suboptimal healing process can cause scar tissue formation, increasing the chance of reinjury [4].
Traditional methods of tendon and ligament repair include suture anchors [5][6] and autogenous tendon grafts [7]. When tendons are divided, the ends can be approximated and sutured to promote healing. Complications such as adhesions [8], repair site gap formation [9], or chondrolysis [10] may occur. Tissue grafting is another well-established method of tendon repair. Autograft involves harvesting tissue from the patient’s own body to replace the damaged tissue, and despite the success rate, complications such as donor site morbidity issues or lack of adequate tissue may arise. Allograft and xenograft involve tissue transplantation from a human or animal, respectively. These have greater availability and flexibility but risk rejection, disease transmission, and zoonotic transmission [11].
In recent years, developments of stem cell therapies have created new avenues for treatment [12]. Induced pluripotent (iPSC) and embryonic stem cells (ESCs) have the highest differentiation capacity and flexibility; however the unlimited regenerative nature of these cells can potentially increase the risk of teratoma development [13] or ectopic bone formation [14]. Mesenchymal stem cells (MSCs) do not have the ethical concerns that surround the use of ESCs but can potentially influence tumorigenesis and lead to cancer treatment resistance [11]. These cells can be used in isolation or with appropriate biomaterial using a suitable scaffolding technique to create the most appropriate environment for tissue regeneration. The purpose of an ideal scaffold should be to seamlessly transition from the artificial alignment of the damaged tissue to the natural regeneration of native tissue using the body’s inherent processes [15].
Recently, there has been a shift in the approach to cell-free therapy, with extracellular components shown to have similar regenerative properties without the potentially harmful effects of entire cell transplantations [16]. Extracellular vesicles (EVs) are extracellular lipid membrane–bound particles that contain host cell-derived protein or nucleic acid messengers, and have an effect on target cells via paracrine or autocrine regulatory functions [17]. This new type of therapy has the potential to change the microenvironment of healing tissue, reducing the inflammatory process and promoting regeneration, as shown in various studies across different organ systems, such as neural [18], musculoskeletal [19], and cardiac [20] tissues.
2. Methodology
An initial review of the literature was performed to gauge the heterogeneity of the literature, after which our search criteria were formulated. This lowered the chance that important studies would be missed.
On 26 May 2021, a systematic search was performed on Embase, Medline, Web of Science, and Cochrane Library, which were considered comprehensive. No filters of any sort were used, and databases were searched from conception. The search strategy is shown in Supplementary Table S1 . All studies found were imported into Mendeley and deduplicated. VL and MT independently completed title and abstract screening and agreement between authors was assessed and generated 93% agreement. A third reviewer (WK) was contacted for unresolvable disagreements. Next, full-text screening was performed by VL and MT, based on the inclusion and exclusion criteria shown in Supplementary Table S2 . Again, a third reviewer (WK) was consulted for any disagreements. A ‘snowball’ search was then performed on 2 June 2021, whereby references of the included studies as well as studies that cited any of the included studies were independently searched by VL and MT, using Google Scholar to identify and screen studies. Studies that performed in vivo experiments, using EVs isolated from human– or animal–derived MSCs, were included. Studies that characterised their MSC population using guidelines from the International Society for Cellular Therapy [21] and characterised their EV population using International Society for Extracellular Vesicles (ISEV) standards were included [22]. In vitro, ex vivo, and in silico studies were excluded, and studies without a control arm were excluded.
Data extraction was independently performed by VL and MT, with a third reviewer (WK) to resolve disagreements. Data were extracted into data tables created in a standardised excel spreadsheet for assessment of study quality and evidence synthesis. Data from each study were split into 4 categories:
- Isolation and characterisation of MSCs, including source of MSCs, cellular origin, cell treatment to extract MSCs, and procedures to verify MSCs (e.g., flow cytometry, western blotting).
- Characterisation and purification of EVs, including MSC purification to extract EVs, EV dimensions, EV biomarkers, imaging used to visualise EVs, and EV active component.
- In vivo model, including method of EV delivery, type of in vivo model, how tendon/ligament injury was induced, animal age, animal weight, animal gender, total number of animals used per experimental group, and follow-up time.
- In vivo findings, including macroscopic appearance, imaging results, histopathological results, biochemical findings, and biomechanical findings.
Quality assessment was carried out independently by MT and VL using the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) tool [23]. The main categories assessed were selection bias, performance bias, blinding bias, attrition bias, and reporting bias. Discrepancies were consulted with WK.
3. Results
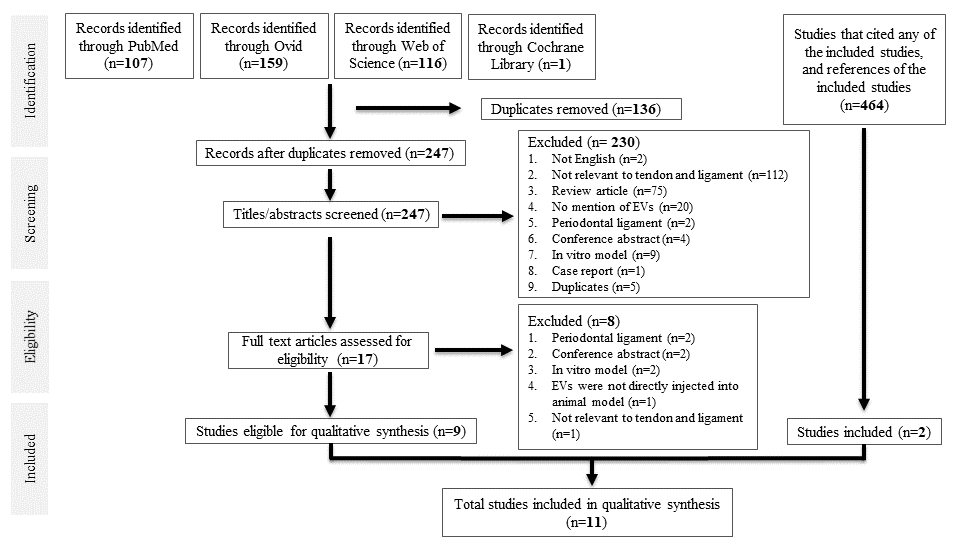
A total of 383 studies were identified from database searching. After de-duplication, 247 studies were identified for title and abstract screening, of which 17 full-text studies were reviewed. Nine studies were eligible for data synthesis. Searching references of the included studies, as well as studies that cited any of the included studies, yielded two more studies, giving a total of 11 studies for qualitative synthesis. All were case–control studies. A PRISMA diagram is shown in Figure 1.
3.1. Characterisation of MSCs
The majority of studies used animal-derived MSCs rather than human-derived MSCs. Of the seven studies that used animal-derived MSCs (involving 310 subjects), the most common MSC donor was Sprague–Dawley rats, with four studies involving 172 subjects utilising them. One study involving 16 subjects used the Lewis rat, and one study involving 32 subjects used NF-_B–luciferase reporter mice. This was done to investigate how MSC-EVs could alter macrophage NF-_B inflammatory signalling. Of the four studies that used human-derived MSCs, involving 138 subjects, two studies involving 93 subjects obtained MSCs from the umbilical cord, one study involving 35 subjects from adipose tissue, and one study involving 10 subjects from bone marrow cells. Regarding the culture medium, alpha-modified minimum essential medium (_-MEM) was most commonly employed, used in seven studies involving 325 subjects. Other culture methods include Dulbecco’s Modified Eagle’s Medium (DMEM) utilised in two studies involving 72 subjects, MesenCult™ Basal Medium utilised in one study involving 16 subjects, and serum-free medium (OriCell) utilised in one study involving 35 subjects.
Regarding the culture medium, alpha-modified minimum essential medium (α-MEM) was most commonly employed, used in seven studies involving 325 subjects. Other culture methods include Dulbecco’s Modified Eagle’s Medium (DMEM) utilised in two studies involving 72 subjects [24][25], MesenCult™ Basal Medium utilised in one study involving 16 subjects [26], and serum-free medium (OriCell) utilised in one study involving 35 subjects [27].
The two most common methods for characterising MSCs were surface-maker expression using flow cytometry (used in seven studies with 310 subjects [28][29][30][31][26][24][25]), and testing for the absence of haematopoietic surface markers CD34 and CD45 (used in five studies involving 226 subjects [28][31][26][24][25]). Another, less common method was trilineage differentiation into adipocytes, osteoblasts, and chondrocytes, seen in four studies involving 140 subjects [30][26][24][25].
The most widespread method of visualising EVs was using transmission electron microscopy (TEM) (used in nine studies involving 372 subjects [28][29][30][31][32][24][33][25][27]), and of those, three studies involving 105 subjects used the method to also measure EV dimensions [24][33][25]. Other methods of visualising EVs included atomic force microscopy (ATM), used in one study involving 16 subjects [26], and an 80kV electron microscope [34]. Additional methods for visualising EVs include tunable resistive pulse sensing (TRPS) using Izon’s qNano Gold in four studies involving 125 subjects [28][29][32][27], nanoparticle tracking analysis (NTA) with ZetaView in three studies involving 202 studies [30][31][34], and AFM in one study [26]. EVs were characterised by flow cytometry and western blotting, with CD9, CD63, TSG-101 being the most common EV markers identified. Gissi et al. attributed the increased extracellular matrix–tendon remodelling to MMP14 and pro-collagen1A2, which were identified in EVs by dot blot [26]. Yao et al. concluded that human umbilical cord-derived MSCs release low levels of miR-21a-3p, which manipulates p65 activity to inhibit tendon adhesion [34].
All studies were designed in a case-control format. Three studies involving 116 subjects divided their experimental subjects into two groups, a control and MSC-EV group [30][32][24]. The rest included multiple experimental groups, with one study involving 35 subjects including a sham surgery group, whereby the tendon would be exposed but not surgically manipulated [27]. One study investigating a dose-dependent relationship between EV concentration and tendon repair further separated their EVs into high (8.4 × 10 12 EVs) and low concentrations (2.8 × 10 12 EVs) [26]. One study utilised hydrogel to promote long-term exosome retention and encourage sustained exosome release, and hence created a separate group that only received hydrogel [31]. Shen et al. compared the efficacy of IFNγ-primed MSC-EVs versus naïve MSC-EVs and hence had three experimental groups in total [29].
3.2 In vivo Findings
Six studies involving 280 subjects performed macroscopic analysis, but one only used it to look for fatty infiltration, confirming the establishment of a rotator cuff tear model. Yu et al. showed that the appearance of the injured tendon better approximated normal tendon after exosome treatment. Two studies involving 100 subjects observed reduced scar formation and two studies involving 93 subjects reported reduced tendon adhesion to peri-tendinous tissue. Histological analysis was performed by all studies. Five studies utilised scoring systems; Shi et al. utilised a fibre alignment score as a proxy for tendon healing. The other four studies involving 161 subjects used histological scores, which includes sub-scores such as fibre structure, cellularity, vascularity, degree of adhesion . Collagen deposition and alignment were assessed by eight studies involving 345 subjects , all of which reported more compact and regularly aligned collagen fibres in EV-treated tendons. One study utilised angiography to show that exosomes promoted angiogenesis around the injury site.
All studies performed biochemical analysis. Four studies involving 227 subjects explicitly mentioned that EVs reduced the expression of pro-inflammatory cytokines such as IL-1_ and IL-6, and increased expression of anti-inflammatory cytokines such as IL- 10 and TGF-_1. Shen et al. demonstrated decreased gene expression and protein expression in the tendon not performed. Three studies involving 154 subjects directly tested the impact of EVs on macrophage polarisation . Huang et al. showed that exosomes decreased CD86, anM1 macrophage surface marker, whilst Shi et al. demonstrated that exosomes decreased iNOS+M1 macrophages and increased Arg1+ M2 macrophages, despite the former being done in vitro. However, Chamberlain et al. reported that exosome treatment had no significant effect on M1 or M2 macrophage number, whilst EV-educated macrophages (made by exposing CD14+ macrophages to MSC-EVs) decreased endogenous M1/M2 macrophage ratio. Virtually all studies reported increased expression of genes related to collagen and tendon matrix formation, such as COL1a1, COL2a1, COL3a1, SCX, Sox9. Gissi et al. also reported a more favourable collagen ratio after EV treatment, i.e., increased collagen type I and decreased collagen type III expression.
Eight studies performed biomechanical analysis. In a bilateral rotator cuff tear model, Wang et al. found that the mean ultimate load to failure in the MSC-EV treated group was significantly greater (132.7 N versus 96 N) than in the control group. In a murine patellar tendon injury model, Yu et al. noticed that stress at the failure of the regenerated tendons and Young’s modulus were 1.84-fold and 1.86-fold higher in the MSC-EV treated group than controls. Most other studies reported that EV treatment increased the maximum stiffness, breaking load, and tensile strength of regenerated tendons; however, three studies reported no significant difference in biomechanical properties between EVtreated and control groups.
3.3. Risk of Bias
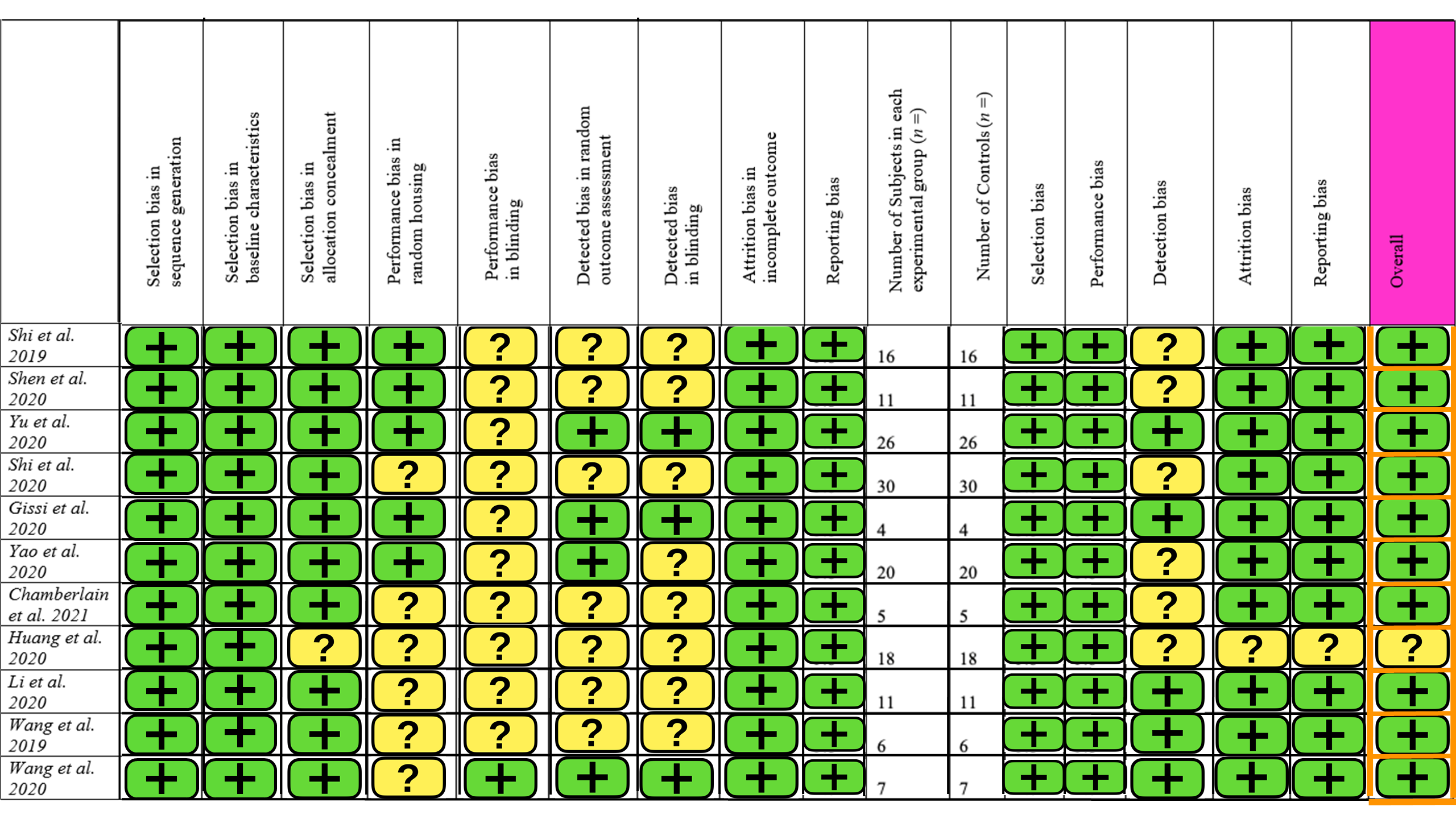
The SYRCLE risk of bias tool for animal studies was used, containing 15 different parameters. A summary of this is provided in Figure 2. Ten studies had a low level of concern overall, but one study had some concern about the risk of bias. The main contributors to bias were blinding and detection bias. There was little selection and reporting bias. Overall, the studies included in this review are of high quality with a low risk of bias.
4. Discussion
4.1. MSC Isolation, Differentiation, and Culture Media
4.2. EV Isolation and Administration
4.3. Modifying EVs to Enhance their Biological Function
Role of MMP-14 and miR-21 in Tendon/Ligament Repair
4.4. EV Educated Macrophages
5. Conclusions
Tendinopathy is a common disorder that results in a significant disease burden. Regenerative approaches via tissue engineering are a promising option, especially novel cell-free therapies utilising MSC-EVs, which have been shown to be effective in in vitro studies. Randomised studies in suitable animal models that mimic human disease are necessary before progression to human trials. In this review, all included in vivo studies reported better tendon/ligament repair following MSC-EV treatment, but not all found improvements in every parameter measured. Although biomechanical properties are very relevant for assessing tendon and ligament healing, this was not consistently assessed. Even if it was assessed, evidence linking biomechanical alterations to functional improvement was weak; studies are needed that rigorously examine the underlying mechanisms for the enhancement of biomechanical properties after MSC-EV treatment. The progression of promising preclinical data to achieve successful clinical market authorisation remains a bottleneck. One hurdle for progress to the clinic is the transition from small animal research to advanced preclinical studies in large animals to test for the safety and efficacy of products. However, it is likely that there will be translational questions not completely answered by animal models as co-morbidities (e.g., obesity, smoking) will be challenging to model. Nevertheless, the studies in this review have showcased the safety and efficacy of MSC-EV therapy for tendon/ligament healing, by attenuating the initial inflammatory response and accelerating tendon matrix regeneration, providing a basis for potential clinical use in tendon/ligament repair.
This entry is adapted from the peer-reviewed paper 10.3390/cells10102553
References
- Markides, H.; Foster, N.; McLaren, J.; Hopkins, T.; Black, C.; Oreffo, R.; Scammell, B.; Echevarria, I.; White, L.; El Haj, A. Short-Term Evaluation of Cellular Fate in an Ovine Bone Formation Model. Cells 2021, 10, 1776.
- Futrega, K.; Music, E.; Robey, P.; Gronthos, S.; Crawford, R.; Saifzadeh, S.; Klein, T.J.; Doran, M.R. Characterisation of ovine bone marrow-derived stromal cells (oBMSC) and evaluation of chondrogenically induced micro-pellets for cartilage tissue repair in vivo. Stem Cell Res. Ther. 2021, 12, 1–19.
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 879–883.
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 2017, 7, 1–13.
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065.
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382.
- Spohn, G.; Witte, A.-S.; Kretschmer, A.; Seifried, E.; Schäfer, R. More Human BM-MSC With Similar Subpopulation Composition and Functional Characteristics Can Be Produced With a GMP-Compatible Fabric Filter System Compared to Density Gradient Technique. Front. Cell Dev. Biol. 2021, 9.
- Hagmann, S.; Moradi, B.; Frank, S.; Dreher, T.; Kämmerer, P.W.; Richter, W.; Gotterbarm, T. Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC Musculoskelet. Disord. 2013, 14, 223.
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the Optimal Culture Conditions for Clinical Scale Production of Human Mesenchymal Stem Cells. Stem Cells 2006, 24, 462–471.
- Dexheimer, V.; Mueller, S.; Braatz, F.; Richter, W. Reduced Reactivation from Dormancy but Maintained Lineage Choice of Human Mesenchymal Stem Cells with Donor Age. PLoS ONE 2011, 6, e22980.
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019, 23, 5475–5485.
- Chamberlain, C.S.; Kink, J.A.; Wildenauer, L.A.; McCaughey, M.; Henry, K.; Spiker, A.M.; Halanski, M.A.; Hematti, P.; Vanderby, R. Exosome-educated macrophages and exosomes differentially improve ligament healing. Stem Cells 2020, 39, 55–61.
- Schop, D.; Janssen, F.W.; Van Rijn, L.D.S.; Fernandes, H.; Bloem, R.M.; De Bruijn, J.D.; Van Dijkhuizen-Radersma, R. Growth, Metabolism, and Growth Inhibitors of Mesenchymal Stem Cells. Tissue Eng. Part A 2009, 15, 1877–1886.
- Scuteri, A.; Donzelli, E.; Rodriguez-Menendez, V.; Ravasi, M.; Monfrini, M.; Bonandrini, B.; Figliuzzi, M.; Remuzzi, A.; Tredici, G. A Double Mechanism for the Mesenchymal Stem Cells’ Positive Effect on Pancreatic Islets. PLoS ONE 2014, 9, e84309.
- Martínez-Lorenzo, M.J.; Cañas, M.R.; Alegre-Aguarón, E.; Desportes, P.; Castiella, T.; García-Álvarez, F.; Larrad, L. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J. Orthop. Res. 2009, 27, 1499–1507.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Yao, Z.; Li, J.; Wang, X.; Peng, S.; Ning, J.; Qian, Y.; Fan, C. MicroRNA-21-3p Engineered Umbilical Cord Stem Cell-Derived Exosomes Inhibit Tendon Adhesion. J. Inflamm. Res. 2020, 13, 303–316.
- Li, J.; Yao, Z.; Xiong, H.; Cui, H.; Wang, X.; Zheng, W.; Qian, Y.; Fan, C. Extracellular vesicles from hydroxycamptothecin primed umbilical cord stem cells enhance anti-adhesion potential for treatment of tendon injury. Stem Cell Res. Ther. 2020, 11, 1–14.
- Wang, C.; Hu, Q.; Song, W.; Yu, W.; He, Y. Adipose Stem Cell–Derived Exosomes Decrease Fatty Infiltration and Enhance Rotator Cuff Healing in a Rabbit Model of Chronic Tears. Am. J. Sports Med. 2020, 48, 1456–1464.
- Nagano, M.; Kimura, K.; Yamashita, T.; Ohneda, K.; Nozawa, D.; Hamada, H.; Yoshikawa, H.; Ochiai, N.; Ohneda, O. Hypoxia Responsive Mesenchymal Stem Cells Derived from Human Umbilical Cord Blood Are Effective for Bone Repair. Stem Cells Dev. 2010, 19, 1195–1210.
- Coipeau, P.; Rosset, P.; Langonné, A.; Gaillard, J.; Delorme, B.; Rico, A.; Domenech, J.; Charbord, P.; Sensebé, L. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy 2009, 11, 584–594.
- Shen, H.; Yoneda, S.; Abu-Amer, Y.; Guilak, F.; Gelberman, R.H. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J. Orthop. Res. 2020, 38, 117–127.
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341.
- Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med. Sci. Monit. 2020, 26.
- Gissi, C.; Radeghieri, A.; Passeri, C.A.L.; Gallorini, M.; Calciano, L.; Oliva, F.; Veronesi, F.; Zendrini, A.; Cataldi, A.; Bergese, P.; et al. Extracellular vesicles from rat-bone-marrow mesenchymal stromal/stem cells improve tendon repair in rat Achilles tendon injury model in dose-dependent manner: A pilot study. PLoS ONE 2020, 15, e0229914.
- Huang, Y.; He, B.; Wang, L.; Yuan, B.; Shu, H.; Zhang, F.; Sun, L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res. Ther. 2020, 11, 1–16.
- Smith, R.P.; Eckalbar, W.L.; Morrissey, K.M.; Luizon, M.; Hoffmann, T.J.; Sun, X.; Jones, S.L.; Aldred, S.F.; Ramamoorthy, A.; Desta, Z.; et al. Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements. PLoS Genet. 2014, 10, e1004648.
- Ryu, A.H.; Eckalbar, W.L.; Kreimer, A.; Yosef, N.; Ahituv, N. Use antibiotics in cell culture with caution: Genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci. Rep. 2017, 7, 1–9.
- Dessels, C.; Potgieter, M.; Pepper, M.S. Making the Switch: Alternatives to Fetal Bovine Serum for Adipose-Derived Stromal Cell Expansion. Front. Cell Dev. Biol. 2016, 4, 115.
- Mesalam, A.; Lee, K.-L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.-H.; Joo, M.-D.; Lee, J.-H.; Jin, J.-I.; Kong, I.-K. A combination of bovine serum albumin with insulin–transferrin–sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases. Reprod. Fertil. Dev. 2019, 31, 333–346.
- Lindroos, B.; Boucher, S.; Chase, L.; Kuokkanen, H.; Huhtala, H.; Haataja, R.; Vemuri, M.; Suuronen, R.; Miettinen, S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 2009, 11, 958–972.
- Shahdadfar, A.; Frønsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In Vitro Expansion of Human Mesenchymal Stem Cells: Choice of Serum Is a Determinant of Cell Proliferation, Differentiation, Gene Expression, and Transcriptome Stability. Stem Cells 2005, 23, 1357–1366.
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1–13.
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell. Vesicles 2019, 8, 1560809.
- Nanoparticle Tracking Analysis vs Tunable Resistive Pulse Sensing. Available online: https://www.izon.com/trps/compare-nta-and-trps (accessed on 10 July 2021).
- Otahal, A.; Kuten-Pella, O.; Kramer, K.; Neubauer, M.; Lacza, Z.; Nehrer, S.; De Luna, A. Functional repertoire of EV-associated miRNA profiles after lipoprotein depletion via ultracentrifugation and size exclusion chromatography from autologous blood products. Sci. Rep. 2021, 11, 1–16.
- Hashimoto, K.; Akagi, M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: A narrative review. J. Int. Med. Res. 2020, 48.
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433.
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 2019, 17, 1–12.
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858.
- Spicer, P.P.; Mikos, A.G. Fibrin glue as a drug delivery system. J. Control. Release 2010, 148, 49–55.
- Lamichhane, T.N.; Jay, S.M. Production of Extracellular Vesicles Loaded with Therapeutic Cargo. Target. Drug Deliv. 2018, 1831, 37–47.
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669.
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 1–11.
- Harting, M.T.; Srivastava, A.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Furman, N.E.T.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90.
- Zayed, M.; Adair, S.; Dhar, M. Effects of Normal Synovial Fluid and Interferon Gamma on Chondrogenic Capability and Immunomodulatory Potential Respectively on Equine Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 6391.
- Gulotta, L.V.; Kovacevic, D.; Montgomery, S.; Ehteshami, J.R.; Packer, J.D.; Rodeo, S.A. Stem Cells Genetically Modified With the Developmental Gene MT1-MMP Improve Regeneration of the Supraspinatus Tendon-to-Bone Insertion Site. Am. J. Sports Med. 2010, 38, 1429–1437.
- Oshiro, W.; Lou, J.; Xing, X.; Tu, Y.; Manske, P.R. Flexor tendon healing in the rat: A histologic and gene expression study. J. Hand Surg. 2003, 28, 814–823.
- Apte, S.S.; Fukai, N.; Beier, D.R.; Olsen, B.R. The Matrix Metalloproteinase-14 (MMP-14) Gene Is Structurally Distinct from Other MMP Genes and Is Co-expressed with the TIMP-2 Gene during Mouse Embryogenesis. J. Biol. Chem. 1997, 272, 25511–25517.
- Kinoh, H.; Sato, H.; Tsunezuka, Y.; Takino, T.; Kawashima, A.; Okada, Y.; Seiki, M. MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J. Cell Sci. 1996, 109, 953–959.
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743.
- Zigrino, P.; Brinckmann, J.; Niehoff, A.; Lu, Y.; Giebeler, N.; Eckes, B.; Kadler, K.E.; Mauch, C. Fibroblast-Derived MMP-14 Regulates Collagen Homeostasis in Adult Skin. J. Investig. Dermatol. 2016, 136, 1575–1583.
- Annabi, B.; Laflamme, C.; Sina, A.; Lachambre, M.-P.; Béliveau, R. A MT1-MMP/NF-κB signaling axis as a checkpoint controller of COX-2 expression in CD133(+) U87 glioblastoma cells. J. Neuroinflamm. 2009, 6, 8.
- Cohen, D.B.; Kawamura, S.; Ehteshami, J.R.; Rodeo, S.A. Indomethacin and Celecoxib Impair Rotator Cuff Tendon-to-Bone Healing. Am. J. Sports Med. 2006, 34, 362–369.
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673.
- Wright, R.W.; Allen, T.; El-Zawawy, H.B.; Brodt, M.D.; Silva, M.J.; Gill, C.S.; Sandell, L.J. Medial collateral ligament healing in macrophage metalloelastase (MMP-12)-deficient mice. J. Orthop. Res. 2006, 24, 2106–2113.
- Chamberlain, C.S.; Leiferman, E.M.; Frisch, K.E.; Wang, S.; Yang, X.; Van Rooijen, N.; Baer, G.S.; Brickson, S.L.; Vanderby, R. The influence of macrophage depletion on ligament healing. Connect. Tissue Res. 2010, 52, 203–211.
- de la Durantaye, M.; Piette, A.B.; van Rooijen, N.; Frenette, J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J. Orthop. Res. 2014, 32, 279–285.
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89.
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20.
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534.
- Chen, S.; Deng, G.; Li, K.; Zheng, H.; Wang, G.; Yu, B.; Zhang, K. Interleukin-6 Promotes Proliferation but Inhibits Tenogenic Differentiation via the Janus Kinase/Signal Transducers and Activators of Transcription 3 (JAK/STAT3) Pathway in Tendon-Derived Stem Cells. Med. Sci. Monit. 2018, 24, 1567–1573.
- Manning, C.N.; Havlioglu, N.; Knutsen, E.; Sakiyama-Elbert, S.E.; Silva, M.; Thomopoulos, S.; Gelberman, R.H. The early inflammatory response after flexor tendon healing: A gene expression and histological analysis. J. Orthop. Res. 2014, 32, 645–652.
- Dakin, S.G.; Werling, D.; Hibbert, A.; Abayasekara, D.R.E.; Young, N.J.; Smith, R.K.W.; Dudhia, J. Macrophage Sub-Populations and the Lipoxin A4 Receptor Implicate Active Inflammation during Equine Tendon Repair. PLoS ONE 2012, 7, e32333.
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells against Sepsis. Stem Cells 2017, 35, 1208–1221.
- Xu, T.; Xu, M.; Bai, J.; Lin, J.; Yu, B.; Liu, Y.; Guo, X.; Shen, J.; Sun, H.; Hao, Y.; et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology 2019, 71, 57–65.
