Capsule-based dry powder inhalers (cDPI) use a hard capsule that contains a powder formulation which consists of a mixture of a micronized drug and a carrier usually the lactose, known for its good lung tolerance. The capsule is either inserted into the device during manufacturer or by the patient prior to use. After perforating, opening or cut the capsule in the device, patients take a deep and rapid breath to inhale the powder, using air as the vector of drug displacement. The system is simple, relatively cheap and characterized by a lower carbon footprint than that of pressurized metered dose inhalers.
- capsule
- dry powder inhaler
- inhalation
- pulmonary drug delivery
1. Introduction

2. Types of Dry Powder Inhalers
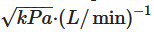 [11]. To produce a fine powder aerosol with increased delivery to the lung, DPIs with low, medium or high intrinsic resistance require inspiratory flows of >90 L/min, 50–60 L/min and <50 L/min, respectively. Notably, due to the increased pressure drop across the device, high resistance DPIs tend to produce a greater lung deposition than those with low intrinsic resistance [11]. Namely, the increase in resistance means that low air flow rates are reached inside the inhaler, and this leads to particles which, given their relative low speed, are less subject to impact mechanisms in the upper airways.
[11]. To produce a fine powder aerosol with increased delivery to the lung, DPIs with low, medium or high intrinsic resistance require inspiratory flows of >90 L/min, 50–60 L/min and <50 L/min, respectively. Notably, due to the increased pressure drop across the device, high resistance DPIs tend to produce a greater lung deposition than those with low intrinsic resistance [11]. Namely, the increase in resistance means that low air flow rates are reached inside the inhaler, and this leads to particles which, given their relative low speed, are less subject to impact mechanisms in the upper airways.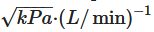 . Because of its low intrinsic resistance, it requires high inspiratory flow rates (100 L/min) to obtain a 4 kPa pressure drop. The flow rate values that are precisely established to standardize the in vitro characterizations of the devices are not always achieved in real life. However, an efficient device must be able to maintain the predetermined performance even at flow rate values around the optimal range. In this regard, Breezhaler delivered consistent doses even to COPD patients who generated a peak inspiratory airflow of approximately 90 L/min through the device [12][13]. In general, patients prefer DPIs with low resistance to those with high resistance [14]. In addition, Janssens et al. [15] have shown that, irrespective of the presence of airway obstruction, 30% and 12.5% of an elderly population were not able to reach the minimum peak inspiratory flow of 45 L/min when using the medium- to high-resistance Turbuhaler DPI and the low-resistance capsule-based DPI Aerolizer. Keeping this in mind, patients would benefit the choice of low resistance DPIs, which are relatively insensitive to variations in peak inspiratory flow at low flow levels about 40–50 L/min. The ERS/ISAM taskforce on inhalers [16] recommends patients “to inhale forcefully from the beginning of inspiration, as deeply as possible, and to continue to inhale for as long as possible”. Indeed, with a DPI, forceful inhalation disperses the micronized drug from the lactose-based carrier into a fine particle dose. The turbulence of the air flow generated in the device is directly related to the resistance of the inhaler and to the flow rate generated by the patient's inhalation act. Turbulence is the driving factor for the deaggregation of the powder and the generation of the fine particle dose.
. Because of its low intrinsic resistance, it requires high inspiratory flow rates (100 L/min) to obtain a 4 kPa pressure drop. The flow rate values that are precisely established to standardize the in vitro characterizations of the devices are not always achieved in real life. However, an efficient device must be able to maintain the predetermined performance even at flow rate values around the optimal range. In this regard, Breezhaler delivered consistent doses even to COPD patients who generated a peak inspiratory airflow of approximately 90 L/min through the device [12][13]. In general, patients prefer DPIs with low resistance to those with high resistance [14]. In addition, Janssens et al. [15] have shown that, irrespective of the presence of airway obstruction, 30% and 12.5% of an elderly population were not able to reach the minimum peak inspiratory flow of 45 L/min when using the medium- to high-resistance Turbuhaler DPI and the low-resistance capsule-based DPI Aerolizer. Keeping this in mind, patients would benefit the choice of low resistance DPIs, which are relatively insensitive to variations in peak inspiratory flow at low flow levels about 40–50 L/min. The ERS/ISAM taskforce on inhalers [16] recommends patients “to inhale forcefully from the beginning of inspiration, as deeply as possible, and to continue to inhale for as long as possible”. Indeed, with a DPI, forceful inhalation disperses the micronized drug from the lactose-based carrier into a fine particle dose. The turbulence of the air flow generated in the device is directly related to the resistance of the inhaler and to the flow rate generated by the patient's inhalation act. Turbulence is the driving factor for the deaggregation of the powder and the generation of the fine particle dose.3. Formulation Aspects of DPI
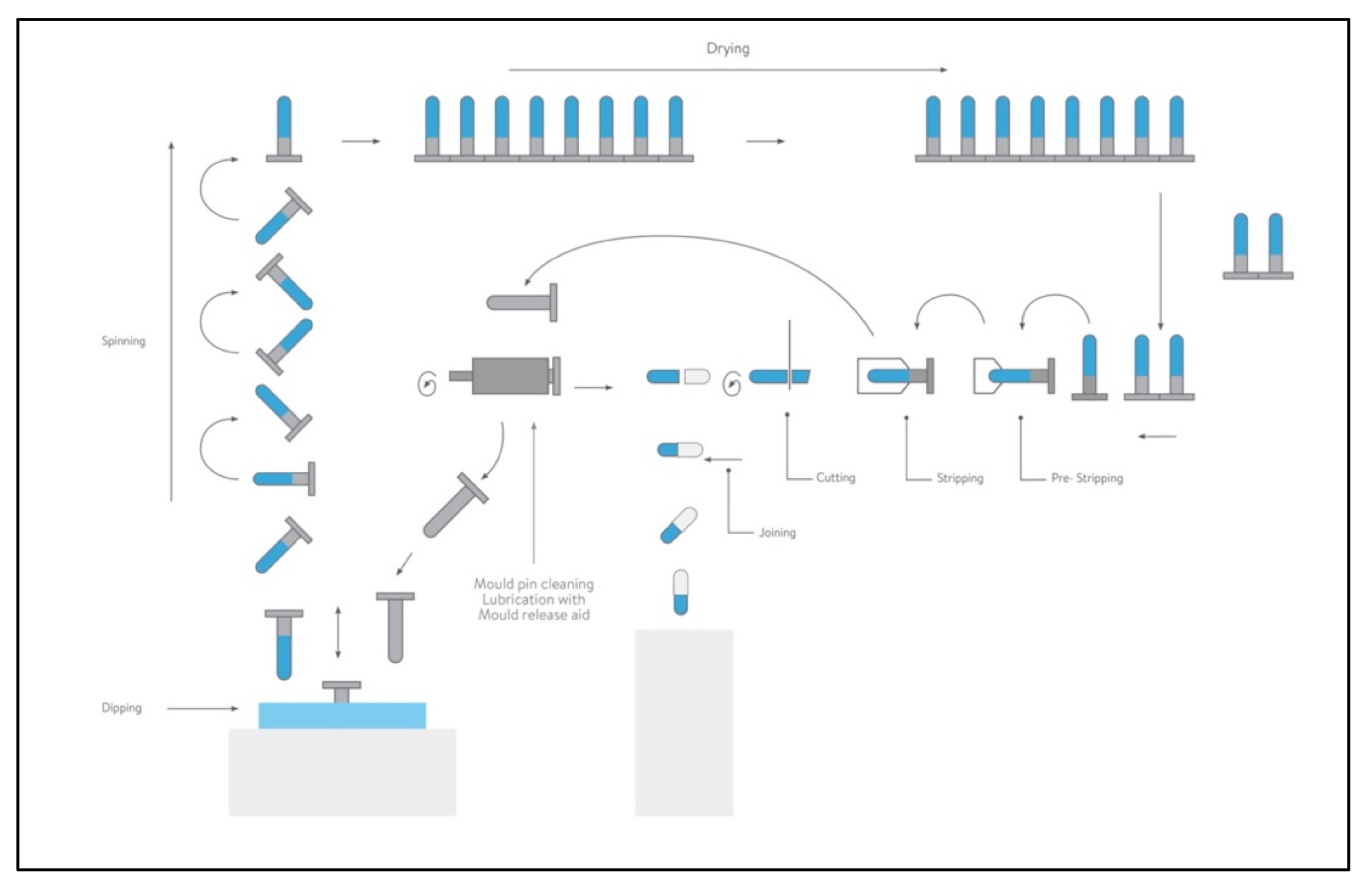
4. Capsule for Inhalation: Composition and Production Aspects
|
Device |
Company |
Commercial Product Name (Drug Delivered) |
Type of Capsule |
|---|---|---|---|
|
Aerolizer® |
Novartis |
Foradil (FF) Foradil Combi (FF, SS) Miflonide (BUD) |
Gelatin |
|
Breezhaler® |
Novartis |
Atectura (IDC, MF) Enerzair (IDC, GPB, MF) Miflonide (BUD) Onbrez (IDC) Seebri (GPB) Ultibro (IDC, GPB) |
HPMC HPMC Gelatin Gelatin Gelatin HPMC |
|
Handihaler® |
Boehringer Ingelheim |
Spiriva (TB) |
Gelatin |
|
Podhaler® |
Mylan |
TOBI (Tobramycin) |
HPMC |
|
Powdair® |
H&T Presspart |
Ventofor Combi Fix (BUD) (FF) |
Gelatin |
|
Rotahaler® |
Cipla |
Asthalin (SS) Budecort (BUD) Duolin (LS-IPB) Duova (TB, FF) Foracort (FF, BUD) Levolin (LS) Seroflo (SX FP) Triohale (ciclesonide, FF, TB) |
- - Gelatin Gelatin Gelatin Gelatin Gelatin Gelatin |
|
RS00 |
Kleva |
Forcap (FF) |
Gelatin |
|
Zentiva |
Formolich (FF) |
||
|
Italchimici |
Kurovent (FF) |
||
|
RS01® |
Adamem |
Zafiron (FF) Fluxiton (FP) |
Gelatin HPMC |
|
Allertec Hellas |
Formaxa (FF) |
Gelatin |
|
|
Baush Health |
Forastmin (FF) |
HPMC |
|
|
Chiesi Farmaceutici |
Bronchitol (Mannitol) |
Gelatin |
|
|
Deva |
Brontio (TB) Foterol (FF) Foterol-B (FF, BDP) Respiro (FP, SX) Rolasym (FF, BUD) Sebraler (GPB) |
Gelatin |
|
|
Exeltis |
Fludalt Duo (FP, SX) Tioumit (TB) |
HPMC Gelatin |
|
|
Galephar Nederland |
Busalair (BUD, SX) |
Gelatin |
|
|
Lek-Am |
Pulmoterol (SX) |
Gelatin |
|
|
Lupin |
Budamate Forte (FF, BUD) Budate (BUD) Duomate (FF, BDP) Esiflo (FP, SX) Formoflo (FF, FP) Lupinhaler (TB) Salbair (LS) Salbair-I (LS, IB) |
Gelatin |
|
|
Polpharma |
Oxodil (FF) |
HPMC |
|
|
Stada |
Formoterol (FF) |
HPMC |
|
|
Spinhaler® |
Aventis |
Sodium cromoglycate |
Gelatin |
|
Turbospin® |
Teva |
Colobreathe (colistimethate sodium) |
Gelatin |
|
Twister® |
Shanghai Sine Promod Pharmaceutical |
Budesonide DPI |
- |
|
Zonda® |
Teva |
Braltus (TB) |
HPMC |
BDP: Beclomethasone dipropionate; BUD: Budesonide; FF: Formoterol fumarate; FP: Fluticasone propionate; GPB: Glycopirronium bromide; IDC: Indacaterol maleate; IP: Ipratropium bromide; LS: Levosalbutamol; MF: Mometasone furoate; SS: Salbutamol sulphate; SX: Salmeterol xinafoate, TB: Tiotropium bromide.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13111936
References
- Lavorini, F.; Buttini, F.; Usmani, O.S. Concepts and Principles of Pharmacology, 100 Years of the Handbook of Experimental Pharmacology. Handb. Exp. Pharm. 2019, 7, 143–159.
- Myrdal, P.B.; Sheth, P.; Stein, S.W. Advances in Metered Dose Inhaler Technology: Formulation Development. AAPS PharmSciTech 2014, 15, 434–455.
- Chierici, V.; Cavalieri, L.; Piraino, A.; Paleari, D.; Quarta, E.; Sonvico, F.; Melani, A.S.; Buttini, F. Consequences of Not-Shaking and Shake-Fire Delays on the Emitted Dose of Some Commercial Solution and Suspension Pressurized Metered Dose Inhalers. Expert Opin. Drug Del. 2020, 17, 1025–1039.
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry Powder Inhalation: Past, Present and Future. Expert Opin. Drug Del. 2016, 14, 499–512.
- Cocozza, S. Inhaling Device for Medial Powder Compositions. U.S. Patent 3807400, 30 April 1974.
- Martinelli, F.; Balducci, A.G.; Rossi, A.; Sonvico, F.; Colombo, P.; Buttini, F. “Pierce and Inhale” Design in Capsule Based Dry Powder Inhalers: Effect of Capsule Piercing and Motion on Aerodynamic Performance of Drugs. Int. J. Pharm. 2015, 487, 197–204.
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for Dry Powder Drug Delivery to the Lung. AAPS PharmSciTech 2015, 16, 479–490.
- Buttini, F.; Balducci, A.G.; Colombo, G.; Sonvico, F.; Montanari, S.; Pisi, G.; Rossi, A.; Colombo, P.; Bettini, R. Dose Administration Maneuvers and Patient Care in Tobramycin Dry Powder Inhalation Therapy. Int. J. Pharm. 2018, 548, 182–191.
- Abadelah, M.; Chrystyn, H.; Bagherisadeghi, G.; Abdalla, G.; Larhrib, H. Study of the Emitted Dose After Two Separate Inhalations at Different Inhalation Flow Rates and Volumes and an Assessment of Aerodynamic Characteristics of Indacaterol Onbrez Breezhaler® 150 and 300 Μg. AAPS PharmSciTech 2018, 19, 251–261.
- Islam, N.; Cleary, M.J. Developing an Efficient and Reliable Dry Powder Inhaler for Pulmonary Drug Delivery–A Review for Multidisciplinary Researchers. Med. Eng. Phys. 2012, 34, 409–427.
- Azouza, W.; Chrystyn, H. Clarifying the Dilemmas about Inhalation Techniques for Dry Powder Inhalers: Integrating Science with Clinical Practice. Prim. Care Resp. J. 2012, 21, 208–213.
- Pavkov, R.; Mueller, S.; Fiebich, K.; Singh, D.; Stowasser, F.; Pignatelli, G.; Walter, B.; Ziegler, D.; Dalvi, M.; Dederichs, J.; et al. Characteristics of a Capsule Based Dry Powder Inhaler for the Delivery of Indacaterol. Curr. Med. Res. Opin. 2010, 26, 2527–2533.
- Colthorpe, P.; Voshaar, T.; Kieckbusch, T.; Cuoghi, E.; Jauernig, J. Delivery Characteristics of a Low-Resistance Dry-Powder Inhaler Used to Deliver the Long-Acting Muscarinic Antagonist Glycopyrronium. J. Drug Assess. 2013, 2, 11–16.
- Palen, J.V.D.; Eijsvogel, M.M.; Kuipers, B.F.; Schipper, M.; Vermue, N.A. Comparison of The Diskus Inhaler And The Handihaler Regarding Preference And Ease of Use. J. Aerosol. Med. 2007, 20, 38–44.
- Janssens, W.; VandenBrande, P.; Hardeman, E.; Langhe, E.D.; Philps, T.; Troosters, T.; Decramer, M. Inspiratory Flow Rates at Different Levels of Resistance in Elderly COPD Patients. Eur. Respir. J. 2008, 31, 78–83.
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.C.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the Pulmonary Specialist Should Know about the New Inhalation Therapies. Eur. Respir. J. 2011, 37, 1308–1417.
- Lavorini, F.; Pistolesi, M.; Usmani, O.S. Recent Advances in Capsule-Based Dry Powder Inhaler Technology. Multidiscip. Resp. Med. 2017, 12, 11.
- Zhou, Q.T.; Tong, Z.; Tang, P.; Citterio, M.; Yang, R.; Chan, H.-K. Effect of Device Design on the Aerosolization of a Carrier-Based Dry Powder Inhaler—A Case Study on Aerolizer® Foradile®. AAPS J. 2013, 15, 511–522.
- Kerwin, E.M.; Spangenthal, S.; Zvarich, M.; Millar, V.; Jain, R.; Collison, K.; Sharma, R. ELLIPTA Versus DISKUS plus HandiHaler in COPD: A Randomized, Open-Label, Crossover Study in a Clinical Trial Setting. Chronic Obs. Pulm. Dis. J. Copd. Found. 2020, 7, 118–129.
- van der Palen, J.; Thomas, M.; Chrystyn, H.; Sharma, R.K.; van der Valk, P.D.; Goosens, M.; Wilkinson, T.; Stonham, C.; Chauhan, A.J.; Imber, V.; et al. A Randomised Open-Label Cross-over Study of Inhaler Errors, Preference and Time to Achieve Correct Inhaler Use in Patients with COPD or Asthma: Comparison of ELLIPTA with Other Inhaler Devices. NPJ Prim. Care Resp. M 2016, 26, 16079.
- Buttini, F.; Rozou, S.; Rossi, A.; Zoumpliou, V.; Rekkas, D.M. The Application of Quality by Design Framework in the Pharmaceutical Development of Dry Powder Inhalers. Eur. J. Pharm. Sci. 2018, 113, 64–76.
- Ding, L.; Brunaugh, A.D.; Stegemann, S.; Jermain, S.V.; Herpin, M.J.; Kalafat, J.; Smyth, H.D.C. A Quality by Design Framework for Capsule-Based Dry Powder Inhalers. Pharm 2021, 13, 1213.
- Quarta, E.; Chierici, V.; Flammini, L.; Tognolini, M.; Barocelli, E.; Cantoni, A.M.; Dujovny, G.; Ecenarro, S.; Sonvico, F.; Colombo, G.; et al. Excipient-Free Pulmonary Insulin Dry Powder: Pharmacokinetic and Pharmacodynamics Profiles in Rats. J. Control. Release Off. J. Control Release Soc. 2020, 323, 412–420.
- Capsugel Site. Available online: http://cpsl-web.s3.amazonaws.com/kc/library/Capsugel_411Inhalation.pdf (accessed on 26 September 2021).
