Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
- probiotics
- inflammasomes
- mammalians
- SARS-CoV-2
1. Introduction
Viral infection triggers host innate immune responses indicated by cytokine production and inflammasome activation [1]. Inflammasomes are cytoplasmic multiprotein complexes which consist of a sensor protein, the adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and the effector protein caspase-1. Active caspase-1 proceeds to cleave the precursors pro-interleukin (IL)-1β and pro-IL-18 into mature forms IL-1β and IL-18, respectively, inducing pyroptosis. Inflammasomes are vital for the maintenance of intestinal homeostasis and gut-associated physiological inflammatory responses in humans. The NLRP3 (NLR family pyrin domain containing 3) inflammasome is assembled in both gut immune and epithelial cells [2] and it is the most widely studied inflammasome.
Probiotic bacteria (PB) are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [3,4]. Lactobacilli and Bifidobacteria are the most common probiotics, but the yeast Saccharomyces boulardii and Bacillus species are also widely known [4]. PB—generally regarded as safe (GRAS)—have beneficial effects in various clinical conditions, such as the prevention of antibiotic-associated diarrhea, constipation, necrotizing enterocolitis, sepsis, and allergies in infants, while recently have shown promising results in oral health and periodontal therapy [5,6,7,8,9,10,11]. Lactic acid bacteria (LAB) can prevent gastrointestinal dysbiosis and reduce the risk of developing secondary infections, while other strains and their metabolites exhibit antiviral activities [12,13], such as enhancement of the barrier function by stimulating mucin secretion, antimicrobial activity by competing with microbial pathogens for nutrients and adhesion to epithelial cells producing antimicrobial substances such as bacteriocins and stimulation of mucosal epithelial cells to secrete defensins. Moreover, PB have immunomodulatory activity by interacting with dendritic cells (DCs), monocytes, and lymphocytes (Figure 1) [14].
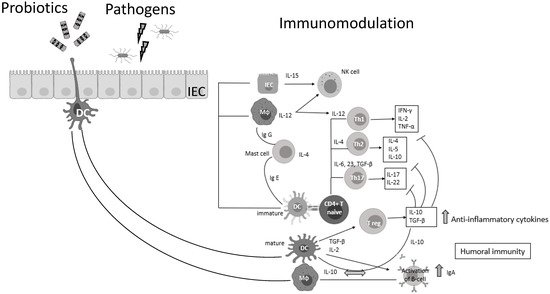
Figure 1. Probiotic bacteria can modulate cytokine release, thus influencing both innate and adaptive immune response. Part of this figure was created with BioRender (https://biorender.com, accessed on 2 October 2021).
It is well documented that PB can regulate inflammation in two ways: (1) indirectly, by producing short-chain fatty acids (SCFAs) and (2) directly, by binding to innate immune system receptors Toll-like (membrane glycoproteins TLR 2, 4, 9) and by triggering important signaling pathways. One of these pathways includes the transcription factor NF-κB, which is associated with the activation of NOD-like receptors (nucleotide-binding oligomerization domain-like receptors), that affect the formation of inflammasomes, thus the inflammatory response [15]. Overactivation of NLRP3 inflammasome has been linked to the pathogenesis of inflammatory bowel disease, cryopyrin-associated periodic syndromes, type 2 diabetes mellitus, atherosclerosis, and neurodegenerative diseases [16]. PB interact with the host by germline-encoded host sensors, namely, pattern recognition receptors (PRRs) [17], that detect microbial structures—pathogen-associated molecular patterns (PAMPs) and components of the host’s cells released during cell damage or death named damage-associated molecular patterns (DAMPs) [18,19]. PRRs are expressed by most innate immune effector cells (dendritic cells, macrophages, monocytes, neutrophils, and epithelial cells). The mechanism of triggering the immune response depends on the kind of antigenic molecule [20]. Many recent studies focus on the complex mechanisms of NLRP3 activation stemming from pathogenic bacteria and their products, such as extracellular ATP and pore-forming toxins (Figure 2).
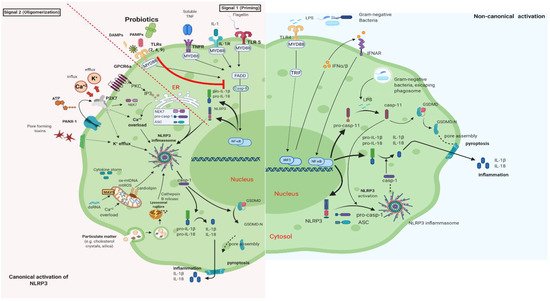
Figure 2. Canonical and Non-Canonical activation of NLRP3 inflammasome. The macrophage in an animal model (transgenic mice-expressing the human angiotensin I-converting enzyme 2 (ACE2) receptor driven by the cytokeratin-18 (K18) gene promoter (K18-hACE2)) [21,22]. (i) The canonical pathway of NLRP3 requires a two-step process: priming and activation. Priming (signal 1) is the upregulation of the NLRP3 inflammasome components, NLRP3, pro-ILβ, and pro-caspase. This transcriptional upregulation is stimulated by PAMPs (bacterial, fungal, viral) or DAMPs which are recognized by PRRs such as TLRs. Additionally, cytokines such as TNF or IL-1 stimulate this upregulation by engaging TNFR and IL1-R, respectively. The activation (signal 2) is provided by various stress signals such as ion or plasma perturbations, ATP, lysosomal rupture, particulate matter, mitochondrial antiviral-signaling protein, mitochondrial reactive oxygen species, ox-mtDNA, etc. Oligomerization of NLRP3 inflammasome activates caspase-1, which in turn cleaves pro-ILβ, pro-IL18, and GSDMD and induces pyroptosis and inflammation [23]. (ii) Gram-negative bacteria (e.g., lipopolysaccharide (LPS) or outer membrane vesicle from bacteria) can activate the non-canonical inflammasome pathway that involves NLRP3-dependent caspase-4/5 in humans (known as caspase-11 in murine models) [24]. Caspase-11, when it is secreted at normal rates, protects against bacterial infections, but its excessive activation can cause tissue damage and pyroptosis which is activated in both pathways through cytosolic gasdermin (GSDMD), a member of the gasdermin family [25]. GSDMD cleavage generates a N-terminal domain that is capable of forming plasma membrane pores and a C-terminal domain that acts as an inhibitor of cytolysis [26]. The cleaved N-terminal domain of GSDMD oligomerizes and forms pores on the host cell membrane, leading to pyroptosis and further activation of NLRP3 by triggering K+ efflux [27,28]. This figure was created with BioRender (https://biorender.com, accessed on 2 October 2021).
2. The Antiviral Activity of Probiotic-Produced Metabolites
PB, through the production of antimicrobial substances, such as bacteriocins (proteinaceous products), inhibit bacterial adherence and invasion capacity in the intestinal epithelium [54]. The inhibitory activity of bacteriocins includes effects against pathogens responsible for hospital-acquired infections and many Gram-negative bacteria. They can bind to the cell surface receptors and reduce the virus-induced cytopathic effects at a preincubation condition. Additionally, bacteriocins inhibit virus replication (Lactobacillus delbrueckii subsp. Bulgaricus 1043 bacteriocin inhibited the replication of influenza virus) blocking receptor sites on host cells and avoid the accumulation of viral particles [14,47,55], as well. Enterocin CRL35, a bacteriocin produced by Enterococcus mundtii CRL35, has proven antiviral effects against strains of Herpes simplex viruses (HSV)-1 and HSV-2 by inhibiting late stages of replication in vitro [56]. Furthermore, probiotic Bacillus amyloliquefaciens produces another bacteriocin, subtilosin, with antiviral properties against HSV and influenza virus. In this context, bacteriocins may have antiviral action against SARS-CoV-2 [57].
Hydrogen peroxide (H2O2) is a defense mechanism with natural microbicide action preventing contamination by microorganisms. H2O2 is a non-proteinaceous substance produced by several bacteria, and probiotics. More specifically, studies showed that Lactobacillus sp. in the vaginal cavity produces H2O2 with toxic effects on viruses like HIV and HSV-2. In vitro tests revealed that the amount of H2O2 was sufficient to inactivate HIV [13,58] and researchers concluded that H2O2 protect against a variety of viral pathogens and improve antigenicity and immunogenicity [59].
In this context, other metabolites produced by Lactobacillus plantarum showed effectiveness against transmissible gastroenteritis virus infection, a member of the family Coronaviridae. However, the evidence on mechanisms of action by which these probiotic-produced metabolites exert their specific effects in certain systems or diseases has not yet been elucidated [59].
2.1. Probiotics Targeting Angiotensin-Converting Enzyme
In animal models experimentally infected with SARS-CoV-2, the protein sequence and structure of ACE2 are conserved across mammalians [60]. However, until now, there has been no published study in which NLRP3 activation was investigated following SARS-CoV-2 infection in animals. LAB fractions (mainly belonging to Lactococcus lactis and Lactobacillus helveticus), in in vitro experiments, showed inhibitory activity towards ACE enzymes by blocking the active sites [24,25]. Moreover, the debris of the dead probiotic cells acts as ACE inhibitors too, suggesting that PB could be a potential blocker of ACE and ACE2 receptors which act as a gateway for SARS-CoV-2 to attack GI cells (Figure 3) [22,61]. Recently, another in vitro trial in this field has confirmed that Lactobacillus plantarum bacteriocins (Plantaricin W, D, and JLA-9) inhibit the entry of SARS-CoV-2 by blocking ACE2 receptors and virus transcription (targeting on the S protein and blocking RNA polymerase (RdRp)) [21]
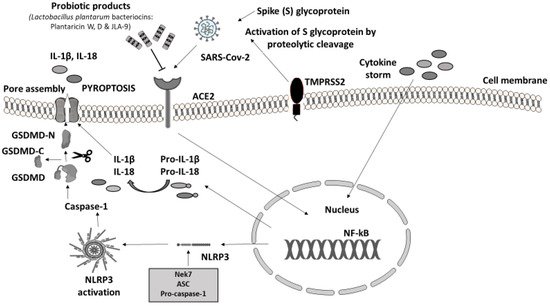
Figure 3. Probiotic bacteria products blocking ACE2 receptors may attenuate COVID-19 infection. Immune response to SARS-CoV-2. Virus invasion is carried out through ACE2 receptor and viral protein S (Spike S glycoprotein) is activated by TMPRSS2 leading to the proinflammatory cytokine cascade. The massive release of cytokines (IL-1β, IL-2, IL-7, IL-8, IFNγ, TNFα, IP10, etc.) from innate immune cells induces the activation of NF-kΒ signaling pathway that regulates the transcription of NLRP3 inflammasome-related components as well as pro-IL-1β and pro-IL-18. As soon as it is formed, NLRP3 inflammasome cleaves pro-caspase-1 and converts it into its active form, caspase-1 [23,62,63]. Subsequently, caspase-1 proceeds with the cleavage of inactive precursors pro-IL-1β and pro-IL-18 to their mature forms IL-1β and IL-18 respectively; at the same time GSDMD is cleaved by the same protease in two domains, an N-terminal and a C-terminal. Following the N-terminal fragment of GSDMD assembles pores in the plasma membrane, inducing pyroptosis and cell death [23]. Experimental studies have shown that SARS-CoV-2 cell entry could be potentially prevented through the antagonistic effect of specific probiotic strains. PB, except for their ability to inhibit the adhesion of pathogens in the intestinal epithelium, compete with pathogens for nutrients and produce antimicrobial substances (e.g., bacteriocins), providing immunomodulatory action and enhancing epithelial barrier function [64]. Their anti-inflammatory effect suppresses cytokine production. Additionally, SARS-CoV-2 evokes inflammation by infecting host cells through the ACE2 receptor and TMPRSS2 inducing proinflammatory cytokine release. Lactobacillus plantarum metabolic product (Plantaricin) blocks the entry of SARS-CoV-2 by binding with ACE2 [21,23,65,66,67]. Parts of this figure were created with BioRender (https://biorender.com, accessed on 2 October 2021) and MOTIFOLIO Download Free Sample (https://www.motifolio.com/sampleslides.html, accessed on 2 October 2021).
2.2. Research Response to the Pandemic
The ability of probiotics to inhibit the virus replication is more effective in the early onset of disease [14]. At present, there are eleven ongoing in vivo studies in this field whilst none of them have been completed (Table 2, Home—ClinicalTrials.gov, assessed on 31 December 2020); thus, the available evidence comes from the laboratory.
Table 2. Ongoing in vivo studies evaluating probiotic treatment in patients with COVID-19 infection (ClinicalTrials.gov accessed on 31 December 2020).
| Number of Clinical Trial | Study Title | Sample Size | Condition/Disease | Group | Interventions | Duration | Measurements | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 04621071 | Efficacy of probiotics in reducing duration and symptoms of COVID-19 | 84 | COVID-19 | Control: Placebo Intervention: 2 probiotic strains |
Placebo: (potato starch and magnesium stearate) Probiotic: (2 strains 10 × 109 CFU |
25 days | Questionnaires (socio-demographic, medical (weight, height, general health, current medication, symptoms), food intake etc.). Optional collection of saliva and stool samples. | Duration of symptoms. Number of days before symptoms disappear. |
| 04458519 | Efficacy of intranasal probiotic treatment to reduce severity of symptoms in COVID-19 infection | 40 | COVID-19 | Control: Placebo Intervention: Probiotics |
Placebo: Saline solution Probiotic: Lactococcus lactis W136 2.4 billion CFU given intranasally |
4 weeks | Visual Analogue Scale (VAS) |
Change in severity of COVID-19 infection. Number of days with any symptom of COVID-19 infection ≥ to 35 as measured on VAS at the 28-day endpoint. |
| 04390477 | Study to evaluate the effect of a probiotic in COVID-19 | 40 | COVID-19 | Control: No treatment Intervention: Probiotics |
Probiotic pill 1 × 109 CFU | 30 days | Patients’ status | Subjects discharged from ICU. |
| 4366180 | Evaluation of the probiotic Lactobacillus Coryniformis K8 on COVID-19 prevention in healthcare workers | 314 | COVID-19 | Control: Placebo Intervention: Probiotics |
Placebo: maltodextrin Probiotic: Lactobacillus K8 per day (3 × 109 CFU/day) |
2 months | SARS-CoV-2 detection by PCR or antigen test | Incidence of SARS-CoV-2 infection in healthcare workers |
| 04666116 | Changes in viral load in COVID-19 after probiotics | 96 | COVID-19 | Control: No dietary administration (probiotics). Medication agreed by the hospital committee Intervention: Medication agreed by the hospital committee and nutritional supplement (probiotics) |
Dietary supplementation with strains from Bifidobacterium longum, Bifidobacterium animalis subsp. Lactis and Lactobacillus rhamnosus | 1 year | SARS-CoV-2 detection by PCR | Viral load during the period of admission |
| 04366089 | Oxygen-Ozone as adjuvant treatment in early control of COVID-19 progression and modulation of the gut microbial flora | 152 | COVID-19 SARS-CoV-2 Pneumonia, Viral Coronavirus Infection |
Control: Drugs (Standard of care) Intervention: Oxygen-ozone, Probiotics and Drugs (Standard of care) |
Drugs: Azithromycin, Hydroxychloroquine Oxygen-ozone therapy + Probiotics Streptococcus thermophilus DSM322245, Bifidobacterium lactis DSM 32246, Bifidobacterium lactis DSM 32247, Lactobacillus acidophilus DSM 32241, Lactobacillus helveticus DSM 32242, Lactobacillus paracasei DSM 32243, Lactobacillus plantarum DSM 32244, Lactobacillus brevis DSM 27961 (200 billion) + Drugs (Azithromycin, Hydroxychloroquine) |
21 days | Delta neutrophil index (Reflects the ratio of circulating immature neutrophils) |
Delta index in the number of patients requiring orotracheal intubation despite treatment |
| 04517422 | Efficacy of L. Plantarum and P. Acidilactisi in adults with SARS-CoV-2 and COVID-19 | 300 | COVID-19 | Control: Placebo Intervention: Probiotics |
Placebo: Combination of maltodextrin (E1400, qs) in a vegetable hydroxymethylpropyl-cellulose capsule Probiotic: Combination of Lactobacillus plantarum CECT7481, Lactobacillus plantarum CECT 7484, Lactobacillus plantarum CECT 7485, and Pediococcus acidilactici CECT 7483 |
30 days | WHO Clinical Progression Scale | Severity progression of COVID-19 |
| 04462627 | Reduction of COVID-19 transmission to health care professionals | 500 | COVID-19 | Intervention 1: COVID-19 positive patients Intervention 2: COVID-19 negative patients Intervention 3: Untested healthy volunteers |
Probiotic (intervention 3) | 3 weeks | Determination of the blood group (ABO/LE) | Anti-A antibody concentration Anti-B antibody concentration Blood group |
| 04420676 | Synbiotic therapy of gastrointestinal symptoms during COVID-19 infection | 108 | COVID-19 | Control: Placebo Intervention: Probiotics |
Placebo: matrix containing maize starch, maltodextrin, inulin, potassium chloride, hydrolysed rice protein, magnesium sulphate, fructooligosaccharide (FOS), enzymes (amylases), vanilla flavour and manganese sulphate. Probiotic: Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Enterococcus faecium W54, Lactobacillus acidophilus W37, Lactobacillus acidophilus W55, Lactobacillus paracasei W20, Lactobacillus plantarum W1, Lactobacillus plantarum W62, Lactobacillus rhamnosus W71 and Lactobacillus salivarius W24 |
30 days | Feces | Duration of diarrhea (defined as days with 3 or more loose stools) |
| 04399252 | Effect of Lactobacillus on the microbiome of household contacts exposed to COVID-19 | 1000 | Microbiome/COVID-19 | Control: Placebo Intervention: Probiotics |
Lactobaciltus rhamnosus GG Placebo Probiotic: Lactobacillus rhamnosus GG |
28 days | Fecal samples | Change in Shannon Diversity Index |
Research, at the time of the pandemic, poses a lot of challenges for academics. The design of the studies was dramatically rapid and, in some cases, with an impact on quality. The studies below enrolled a small number of participants, whilst heterogeneity was observed in measurements of the outcomes. Another possible drawback is the use of one probiotic strain in some interventions. Multispecies PB seems to be more effective compared to monostrain probiotics. When multispecies PB are combined, they act synergistically and can have a significantly positive effect. Their activity can also be stimulated through symbiosis among different strains [68].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9112376
This entry is offline, you can click here to edit this entry!
