Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Meibomian gland dysfunction (MGD) is the leading cause of dry eye disease and loss of ocular surface homeostasis. Increasingly, several observational clinical studies suggest that dyslipidemia (elevated blood cholesterol, triglyceride, or lipoprotein levels) can initiate the development of MGD.
- dyslipidemia
- meibomian gland dysfunction
1. Introduction
The meibomian gland (MG) is a modified sebaceous gland in the eyelids which produces meibum, the lipid component of the tear film [1]. Meibum is essential for retarding tear film evaporation, thereby preventing dry eye [1]. Meibum has the ability to lower surface tension, thus aiding in the spreading of the tear film at the ocular surface [2]. Additionally, it possesses some antimicrobial properties believed to prevent ocular surface infections [3].
Regulation of the MG is complex and thought to involve autonomic nervous control and hormonal influences [1] (Figure 1). The MG is the only sebaceous gland in the body that is rich in sensory, sympathetic, and parasympathetic innervation. It has been shown to express neurotransmitters and neuropeptides including serotonin, calcitonin gene-related peptide, neurotensin, somatostatin, neuropeptide-Y and γ-aminobutyric acid. It also expresses mRNAs for cholinergic, dopaminergic, glutaminergic and adrenergic receptors [1][4][5]. However, it is unclear how these neurochemicals are released around the MG and how they exert their physiological effects on the MG. Regarding hormonal control of the MG, a few studies suggest that androgenic hormones and insulin may be necessary for the growth and normal functioning of the MG [1][2][3][6].
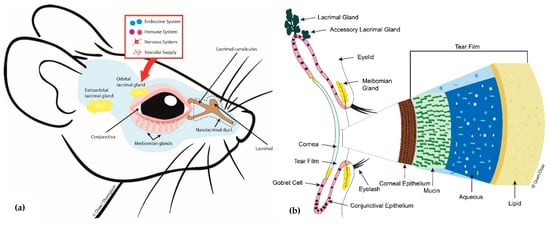
Figure 1. Diagram showing (a) mouse meibomian glands (lower eyelid only) and related ocular surface structures and (b) a cross-sectional view of some key lacrimal functional unit (LFU) components, the tear film components including the outermost lipid produced by the meibomian gland (MG) and how it interacts with the corneal surface.
As a contributor to the precorneal tear film, the MG is an integral component of the lacrimal functional unit and the ocular surface; together with the cornea, the conjunctiva, main and accessory lacrimal glands (Figure 1) [7]. Pathology of the MG, clinically termed meibomian gland dysfunction (MGD), is the leading cause of dry eye disease [8][9]. MGD is known to be present in up to 70 percent of dry eye cases seen globally, and in the United States alone, MGD affects over 7 million people [1][10].
The international workshop on meibomian gland dysfunction defined MGD as a chronic, diffuse abnormality of the MG and may be characterized by terminal duct obstruction and quantitative and qualitative changes in the glandular secretion [1][11]. These can result in alterations of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease [1]. MGD exists in two broad categories: as low delivery and high delivery forms [1]. Low delivery MGD is further divided into hyposecretory and obstructive types, where reduced volume of meibum secretion results from glandular dropout and/or blockage of MG orifices, respectively [12][13]. Higher delivery or hypersecretory forms of MGD are characterized by a large volume of meibum at the lid margin on gentle application of pressure on the tarsus. Hypersecretory MGD is often associated with seborrheic dermatitis, hence it is also referred to as seborrheic MGD [12][13].
This subtype of MGD has been linked to acne rosacea, where skin sebocytes produce excess sebum. Since the MG is a modified sebaceous gland, the proposed explanation is that the seborrhea may be due to an end-organ hyper-response of the MG to androgens [1][14]. On one hand, reduction in levels of some androgens like testosterone, dehydroepiandrosterone and its conjugated dehydroepiandrosterone sulfate are considered as potential risk factors for developing MGD [15]. Other well-known risk factors include desiccative stress at the ocular surface due to low humidity environments [16]. To date, the etiology of MGD is not fully understood, but aging, prolonged contact lens wear, recurrent lid infections, use of certain drugs (e.g., isotretinoin, chemotherapy) and irradiation are known risk factors for developing MGD [17][18][19][20][21].
Dyslipidemia has also been linked to the development of MGD, but direct evidence supporting this relationship is lacking [22][23][24][25]. Dyslipidemia is a disorder of systemic lipid metabolism which is characterized by (abnormal) increased levels of total blood cholesterol (TC), triglycerides (TGs), low-density lipoproteins (LDL) and/or a reduction in high-density lipoproteins (HDL). It is a major component of the metabolic syndrome which includes abdominal obesity, systemic inflammation, insulin resistance, hypertension and hyperglycemia [26][27].
Dyslipidemia is a major risk factor for heart disease, but exactly how it might contribute to MGD is unknown [28][29][30][31]. Plasma lipids have been suggested to affect the MG as well, but the evidence is largely circumstantial [22][23][24][31][32]. Moreover, dating back to 1957, one can find reports stating that there is no relationship between the status of plasma lipids and MG lipid composition [1][22][23][24][30][31][32]. The idea that plasma lipids can affect the MG is anecdotal and likely based on the fact that it is a lipid-synthesizing organ and alterations in systemic lipid metabolism could affect its structure and function [22][23][24][31][32]. Whether pathologic levels of systemic lipids contribute to MGD remains speculative [1][33]. It is thus important to determine if experimental evidence will support a link between dyslipidemia and MGD. This takes on added significance given the global epidemic status of the metabolic syndrome—which affects approximately 25% of the adult population and shows increasing incidence in children [26][27][28].
2. Blood Lipid Profiles and MGD; Study Limitations and Confounders
The relationship between plasma lipid status and meibomian gland health remains unclear and warrants investigation [1][30]. Therefore, the role of dyslipidemia in the development of MGD remains a grey area [1][25][34]. In spite of this, these studies provide useful insights into the potential effects of dysregulated systemic lipid metabolism on the MG, with shared similarities in their approach to examine whether a relationship exists between MGD and dyslipidemia [22][23][24][29][30].
The most relevant observation across all but one of the studies was the finding that patients with MGD had a high prevalence of dyslipidemia relative to controls [22][24][31][32]. This was contrasted by results in one of the studies where prevalence of dyslipidemia among patients with MGD was no different from those without MGD [22]. In fact, for this particular study, the prevalence ratio for dyslipidemia among cases and controls was ~1.0, leading the authors to speculate that dyslipidemia might not be the sole driving factor for the development of MGD [23].
While this was the case for this individual study [22], the other studies reported significant associations between elevated levels of blood TCs, TGs, LDLs, and decreased levels of HDL [22][31][32]. Further, two of the studies reported a significant association between MGD and elevated (> 40 mg/dL) blood levels of HDL [31][32]. Similarly, in one of the studies where specific tests of association between elevated HDL and MGD were not performed, the investigators concluded that elevated HDL may have a unique role in the development of MGD [24]. This was based on the observation that study participants with moderate to severe MGD showed increased levels of HDL [24]. What draws our attention is that in many metabolism-related studies, elevated HDL levels tend to be beneficial and usually not associated with pathologic states. This is because HDL, also known as “good cholesterol”, works to clear systemic circulation of “bad cholesterol” (LDL), thus reducing the risk for cardiovascular disease [35][36][37][38]. Whether high levels of HDL are selectively detrimental to the MG is yet to be explored [24][25][32].
Further, MGD is a multifactorial condition, making it challenging to conclude that the findings from these studies are largely attributable to dyslipidemia. Ageing, prolonged contact lens wear, and adverse environmental conditions including desiccating stress and the use of certain drugs like isotretinoin are known to negatively affect the MG [1][16][18]. Other factors that can influence the development of MGD include autoimmune conditions like Sjögren’s syndrome, ocular graft-versus-host diseases and, rosacea and hormonal insufficiencies [39][40][41].
Collectively, these studies underscore the need to better understand the relationship between dyslipidemia and MGD. Even though some of these studies report significant associations between MGD, age and sex of participants [22][23][24][31], little or no information is provided about how these variables may interact with other risk factors in the development of MGD. For example, only Pinna et al. [32] commented on the contact lens wear status of a few of the subjects; however, it was uncertain how this observation could impact their findings. Further, it has widely been published that ageing affects the MG by decreasing meibocyte differentiation, cell cycling and impaired peroxisome proliferator-activated receptor (PPAR)–γ signaling, leading to reduced secretory activity [42]. On its own, ageing is a significant risk factor for impaired metabolism, hence dyslipidemia [24][43][44]. Therefore, in drawing conclusions about the link between MGD and dyslipidemia, it is critical to apply an analytical model which considers the complex interactions between, e.g., age, sex and dyslipidemia and how these could separately or synergistically affect the MG.
Overall, while these studies provide useful information about the potential involvement of dyslipidemia in the pathogenesis of MGD, they carry several limitations. The studies are observational in nature with similar clinical settings, thus making it impossible to establish a “cause and effect” relationship [22][23][24][31][32]. Moreover, all five studies admitted a challenge with the sample characteristics. In the study by Dao et al. [24] for instance, historical controls obtained from the NHANES were slightly younger (mean age = 52.2 years) than actual cases studies (mean age = 46.6 years). According to the investigators, this could have contributed to the observed increase in the prevalence of hypercholesterolemia among the cases compared to the controls, given that old age is a risk factor for both MGD and impaired lipid metabolism [1][24].
Some of the studies acknowledge limitations with sample size, recommending larger studies to definitively probe if any relationship exists between MGD and dyslipidemia [22][24][31][32]. However, considering the fact that the majority of these studies were restricted to homogeneous groups of participants of solely Indian or Italian ethnicity, future larger population-based studies should aim at including participants of diverse ethnic backgrounds [22][31][32]. This will be very important in strengthening the observed associations and the generalizability of conclusions.
To date, only these few studies have come close in their attempts to investigate the relationship between dyslipidemia and MGD [22][23][24][31][32]. They provide some useful information about the potential link between MGD and dyslipidemia, but the considerable challenges with their study designs merit further prospective studies, especially at the basic science level [25]. This makes it useful to study this relationship in an experimental animal model, where dyslipidemia could be induced and controlled and the corresponding effects on the MG examined through lipidomics and imaging.
Consequently, the next sub-sections of this review are focused, albeit briefly, on discussing experimental prospects regarding this subject. A diet-induced obesity model (DIO), where mice fed a high-fat diet develop dyslipidemia, is introduced, and the power of lipidomics to enhance our understanding of the relationship between dyslipidemia and MGD is considered [45][46].
This entry is adapted from the peer-reviewed paper 10.3390/ijms20143505
References
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978.
- Millar, T.J.; Schuett, B.S. The real reason for having a meibomian lipid layer covering the outer surface of the tear film–A review. Exp. Eye Res. 2015, 137, 125–138.
- Mudgil, P. Antimicrobial role of human meibomian lipids at the ocular surface. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7272–7277.
- Cox, S.M.; Nichols, J.J. The neurobiology of the meibomian glands. Ocul. Surf. 2014, 12, 167–177.
- Seifert, P.; Spitznas, M. Vasoactive intestinal polypeptide (VIP) innervation of the human eyelid glands. Exp. Eye Res. 1999, 68, 685–692.
- Schirra, F.; Richards, S.M.; Liu, M.; Suzuki, T.; Yamagami, H.; Sullivan, D.A. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp. Eye Res. 2006, 83, 291–296.
- Stern, M.E.; Beuerman, R.W.; Fox, R.I.; Gao, J.; Mircheff, A.K.; Pflugfelder, S.C. A unified theory of the role of the ocular surface in dry eye. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2; Springer: Boston, MA, USA, 1998; pp. 643–651.
- Bron, A.; Tiffany, J. The contribution of meibomian disease to dry eye. Ocul. Surf. 2004, 2, 149–164.
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92.
- Bron, A.J.; De Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Uchino, Y. Tfos dews II pathophysiology report. Ocul. Surf. 2017, 15, 438–510.
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937.
- Schaumberg, D.A.; Nichols, J.J.; Papas, E.B.; Tong, L.; Uchino, M.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1994–2005.
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Tomidokoro, A.; Amano, S. Proposed diagnostic criteria for seborrheic meibomian gland dysfunction. Cornea 2010, 29, 980–984.
- Shine, W.E.; McCulley, J.P. Association of meibum oleic acid with meibomian seborrhea. Cornea 2000, 19, 72–74.
- Tamer, C.; Oksuz, H.; Sogut, S. Androgen status of the nonautoimmune dry eye subtypes. Ophthalmic Res. 2006, 38, 280–286.
- Suhalim, J.L.; Parfitt, G.J.; Xie, Y.; De Paiva, C.S.; Pflugfelder, S.C.; Shah, T.N.; Jester, J.V. Effect of desiccating stress on mouse meibomian gland function. Ocul. Surf. 2014, 12, 59–68.
- Arita, R.; Itoh, K.; Inoue, K.; Kuchiba, A.; Yamaguchi, T.; Amano, S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009, 116, 379–384.
- Moy, A.; McNamara, N.A.; Lin, M.C. Effects of isotretinoin on meibomian glands. Optom. Vis. Sci. 2015, 92, 925–930.
- Eom, Y.; Baek, S.; Kim, H.M.; Song, J.S. Meibomian gland dysfunction in patients with chemotherapy-induced lacrimal drainage obstruction. Cornea 2017, 36, 572–577.
- Woo, Y.J.; Ko, J.; Ji, Y.W.; Kim, T.I.; Yoon, J.S. Meibomian gland dysfunction associated with periocular radiotherapy. Cornea 2017, 36, 1486–1491.
- Westekemper, H.; Anastassiou, G.; Sauerwien, W.; Chauvel, P.; Bornfeld, N.; Steuhl, K.P.; Meller, D. Analysis of ocular surface altertions following proton beam radiation in eyes with conjuctival malignant melanoma. Ophthamologe 2006, 103, 858–895.
- Braich, P.S.; Howard, M.K.; Singh, J.S. Dyslipidemia and its association with meibomian gland dysfunction. Int. Ophthalmol. 2016, 36, 469–476.
- Bukhari, A.A. Associations between the grade of meibomian gland dysfunction and dyslipidemia. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 101–103.
- Dao, A.H.; Spindle, J.D.; Harp, B.A.; Jacob, A.; Chuang, A.Z.; Yee, R.W. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am. J. Ophthalmol. 2010, 150, 371–375.
- Kuriakose, R.K.; Braich, P.S. Dyslipidemia and its Association with Meibomian Gland Dysfunction: A Systematic Review. Int. Ophthalmol. 2018, 38, 1809–1816.
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438.
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Sherwin, R.S. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374.
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689.
- Tietge, U.J. Hyperlipidemia and cardiovascular disease: Inflammation, dyslipidemia, and atherosclerosis. Curr. Opin. Lipidol. 2014, 25, 94–95.
- Van Haeringen, N.; Glasius, E. Cholesterol in human tear fluid. Exp. Eye Res. 1975, 20, 271–274.
- Guliani, B.P.; Bhalla, A.; Naik, M.P. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J. Ophthalmol. 2018, 66, 1411–1416.
- Pinna, A.; Blasetti, F.; Zinellu, A.; Carru, C.; Solinas, G. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology 2013, 120, 2385–2389.
- Shine, W.E.; McCulley, J.P. The role of cholesterol in chronic blepharitis. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2272–2280.
- Barter, P.; Rye, K.A. High density lipoproteins and coronary heart disease. Atherosclerosis 1996, 121, 1–12.
- Modulo, C.M.; Filho, E.B.M.; Malki, L.T.; Dias, A.C.; De Souza, J.C.; Oliveira, H.C.; Rocha, E.M. The role of dyslipidemia on ocular surface, lacrimal and meibomian gland structure and function. Curr. Eye Res. 2012, 37, 300–308.
- Bowe, B.; Xie, Y.; Xian, H.; Balasubramanian, S.; Al-Aly, Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 2016, 89, 886–896.
- Bowe, B.; Xie, Y.; Xian, H.; Balasubramanian, S.; Zayed, M.A.; Al-Aly, Z. High density lipoprotein cholesterol and the risk of all-cause mortality among US veterans. Clin. J. Am. Soc. Nephrol. 2016, 11, 1784–1793.
- Ko, D.T.; Alter, D.A.; Guo, H.; Koh, M.; Lau, G.; Austin, P.C.; Wijeysundera, H.C. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: The CANHEART study. J. Am. Coll. Cardiol. 2016, 68, 2073–2083.
- Sullivan, D.A.; Sullivan, B.D.; Evans, J.E.; Schirra, F.; Yamagami, H.; Liu, M.; Dana, M.R. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann. N. Y. Acad. Sci. 2002, 966, 211–222.
- Sullivan, B.; Cermak, J.; Sullivan, R.; Papas, A.; Evans, J.; Dana, M.R.; Sullivan, D.A. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjögren’s syndrome. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3; Springer: Boston, MA, USA, 2002; pp. 441–447.
- Engel, L.; Wittig, S.; Bock, F.; Sauerbier, L.; Scheid, C.; Holtick, U.; Steven, P. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplant. 2015, 50, 961–967.
- Nien, C.J.; Massei, S.; Lin, G.; Nabavi, C.; Tao, J.; Brown, D.J.; Jester, J.V. Effects of age and dysfunction on human meibomian glands. Arch. Ophthalmol. 2011, 129, 462–469.
- Le Couteur, D.G.; Cogger, V.C.; McCUSKEY, R.S.; De Cabo, R.; SmedsrØd, B.; Sorensen, K.K.; Fraser, R. Age-related changes in the liver sinusoidal endothelium: A mechanism for dyslipidemia. Ann. N. Y. Acad. Sci. 2007, 1114, 79–87.
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52.
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid–protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993.
- Butovich, I.A.; Wojtowicz, J.C.; Molai, M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J. Lipid Res. 2009, 50, 2471–2485.
This entry is offline, you can click here to edit this entry!
