Poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) has high optical transparency in the visible light range and low-temperature processing condition, making it one of the most widely used polymer hole transport materials inverted perovskite solar cells (PSCs), because of its high optical transparency in the visible light range and low-temperature processing condition. However, the stability of PSCs based on pristine PEDOT:PSS is far from satisfactory, which is ascribed to the acidic and hygroscopic nature of PEDOT:PSS, and property differences between PEDOT:PSS and perovskite materials, such as conductivity, work function and surface morphology. This review summaries recent efficient strategies to improve the stability of PEDOT:PSS in PSCs and discusses the underlying mechanisms. This review is expected to provide helpful insights for further
increasing the stability of PSCs based on commercial PEDOT:PSS.
1. Introduction
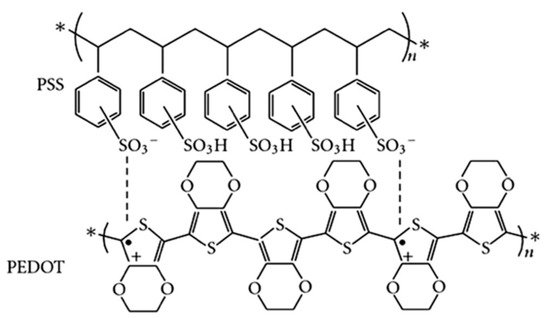
Poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS, chemical structure is shown in
Figure 1) is the most successful conducting polymer, which has been widely used in displays, transistors, various sensors and photovoltaics (PVs) [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. It can be dispersed in water as well as some organic solvents and conventional solution based coating methods can be used to fabricate high-quality PEDOT:PSS films [
18,
19]. PEDOT:PSS films are uniform and highly transparent in the visible range. The electrical conductivity of PEDOT:PSS film can be adjusted within 10
−2 to 10
3 S/cm with certain synthetic conditions, the utilization of different additives or post-treatment methods [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36]. Furthermore, PEDOT:PSS is a low cost material with excellent thermal stability and high mechanical flexibility. Therefore, in recent years, PEDOT:PSS is the most popular hole transport layer (HTL) used in inverted perovskite solar cells (PSCs) [
37,
38,
39].
Figure 1. Chemical structure of PEDOT:PSS. n: degree of polymerization, *: repeated structural units, +: positive charge, •: negative charge.
Perovskite solar cells receive much attention as a next-generation solar technology for energy harvesting due to their very impressive energy conversion efficiency with low fabrication cost [
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66]. Power conversion efficiencies (PCE) of PSCs are increased from 3.8% in 2009 up to the current world record of 25.6% [
67,
68]. However, the serious long-term instability of PSCs limits their commercialization. The stability of PSCs, especially under ambient ultraviolet radiation and humidity, is one of the major drawbacks recently addressed by the photovoltaic scientific community. For PSCs devices, HTL is usually indispensable for effectively blocking electrons and transporting holes. In addition, it affects the quality of the upper perovskite layer which directly affects the efficiency and stability of the devices [
69,
70].
Most inverted PSCs using PEDOT:PSS as the HTL material due to the low temperature processability and simple solution-process ability.
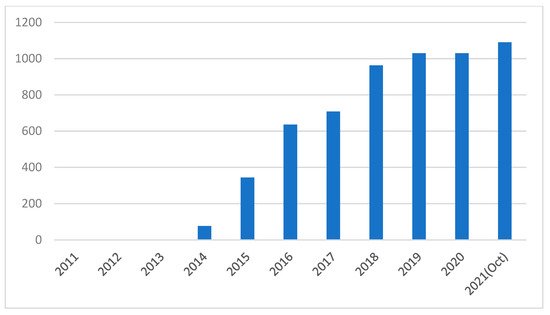
Figure 2 shows the increment in the number of research papers published in recent years on PSCs using PEDOT:PSS as the HTL. The increased research indicates that PEDOT:PSS is a promising HTL material in PSCs. However, the use of PEDOT:PSS would affect the stability of cells due to its hygroscopic and acidic nature [
71]. The acidic nature of PEDOT:PSS will corrode the ITO electrode. Moreover, the hygroscopic nature of PEDOT:PSS results in moisture absorption from the environment which causes decomposition of the perovskite absorber layer [
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74]. Some p-type inorganic materials, including CuSCN, CuI and NiO, have been proposed as promising alternatives to PEDOT:PSS for enhancing the stability of PSCs [
75,
76,
77]. However, the low conductivity of inorganic materials limits the performance of PSCs. Therefore, optimizing the properties of the PEDOT:PSS HTL is crucial in fabricating PSCs with long-term stability. It has been shown that optimization of the PEDOT:PSS HTL layer, such as the pH value, hydrophilicity, work function, surface morphology and electrical conductivity of PEDOT:PSS can improve PCE and stability of PSCs [
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102,
103,
104,
105,
106,
107,
108].
Figure 2. Number of articles published per year on PSCs using PEDOT:PSS [
109].
2. Methods to Improve the PSCs Stability by Tailoring PEDOT:PSS HTL
Many efforts have been made to the modification of the PEDOT:PSS layer for improving the long-term stability of PSCs [
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102].
Table 1 lists the PCE and long-term stability of PSCs adopting PEDOT:PSS as HTL in previous research work. Generally, modification methods can be classified into three types: doping [
61,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93,
94], post-treatment [
62,
95,
96,
97] and using bilayer [
98,
99,
100,
101,
102]. Furthermore, there are some other methods reported to modify the properties of PEDOT:PSS for improving the device stability, such as, using other dopants to replace PSS [
56,
103], and developing new processing methods of PEDOT:PSS film [
104,
105].
Table 1. The long-term stability of PSCs with PEDOT:PSS as HTL.
|
Method
|
Materials
|
Perovskite Materials
|
PCE (%)
|
Stability
|
Ref.
|
|
Doping
|
Imidazole
|
MAPbI3
|
15.7%
|
75% for 14 days, 20% humidity
|
[78]
|
|
CuSCN /NH3 (aq)
|
MAPbI3
|
15.3%
|
71% for 175 h
|
[79]
|
|
Ammonia
|
MAPbI3-xClx
|
13.38%
|
90% for 30 days in N2
|
[80]
|
|
Urea
|
MAPbI3
|
18.8%
|
97% for 10 days, 35% humidity
|
[81]
|
|
metal oxides
|
MAPbI3
|
19.64%
|
90% for 45 days in N2, 80% for 20 days in air
|
[82]
|
|
Dopamine
|
MAPbI3
|
16.4%
|
85.4% for 28 days
|
[83]
|
|
F4-TCNQ
|
MAPbI3-xClx
|
17.22%
|
75% for 150 h, 40% humidity
|
[84]
|
|
DMSO
|
MAPbI3
|
16.7%
|
83% for 590 h
|
[85]
|
|
Nafion
|
MAPbI3
|
16.72%
|
86.6% for 500 h, 30–50% humidity
|
[86]
|
|
graphene flakes
|
MAPbI3
|
4%
|
Stable for one weak
|
[87]
|
|
PSSNa
|
MAPbI3
|
15.56%
|
>85% for 60 days in N2,
|
[88]
|
|
PFI
|
FA0.6MA0.4Sn0.6Pb0.4I3
|
15.85%
|
Stable for 300 s
|
[89]
|
|
Triton X-100
|
MAPbI3
|
16.23%
|
80% for 500 h
|
[90]
|
|
CTAB
|
MAPbI3
|
12.53%
|
75% for 30 days, 20–40% humidity
|
[91]
|
|
SBS
|
MA0.8FA0.2PbI3-xClx
|
19.41%
|
90% for 20 days
|
[92]
|
|
EMIC ionic liquid
|
MAPbI3
|
20.06%
|
85% for 35 days, 60% humidity, 87% after 80 °C for 24 h
|
[93]
|
|
Zn
|
MAPbI3
|
13.2%
|
91% for 168 h
|
[94]
|
|
RbCl
|
MA0.7FA0.3Pb(I0.9Br0.1)3
|
18.3%
|
78.17% for 120 h, 50% humidity
|
[61]
|
|
Post-Treatment
|
GO
|
MAPbI3
|
15.34%
|
83.5% for 39 days, 15% humidity
|
[95]
|
|
WOx doped, EG treated
|
MAPbI3Cl3-x
|
12.69%
|
thermal stable at 250 °C
|
[96]
|
|
EG and MeOH
|
MAPbI3
|
18.18%
|
65% for 350 h, 45% humidity
|
[97]
|
|
Water
|
MAPbI3-xClx
|
18.0%
|
50% for 240 h in air
|
[62]
|
|
Bilayer
|
V2O5
|
MAPbI3
|
15%
|
95% for 18 days
|
[98]
|
|
VOx
|
MAPbI3
|
14.22%
|
77% for 15 days, 40% humidity
|
[99]
|
|
NPB
|
MAPbI3
|
18.4%
|
70% for 20 days, 30±5% humidity
|
[100]
|
|
SrGO
|
MAPbI3
|
16.01%
|
85% for 30 days
|
[101]
|
|
MI
|
FA0.2MA0.8PbI3-xClx
|
20.68%
|
80% for 600 h, 50% humidity
|
[102]
|
3. Other Methods to Improve the PSCs Stability by Tailoring PEDOT:PSS Layer
Many other ameliorative techniques have been developed to improve the device stability using various approaches. Since the aforementioned drawbacks of PEDOT:PSS are almost all related to PSS, some groups focus on exploring other dopants to replace PSS. Jiang et al. reported a facile solid-state synthesis of conducting polymer PEDOT through an in-situ solid-state polymerization from an inexpensive monomer 2,5-dibromo-3,4-ethylenedioxythiophene (DBEDOT) [
103]. The devices using DBEDOT as HTL remained over 80% of their initial PCEs after 720 h storage time. Yu et al. reported a new PEDOT-based HTL adopting sulfonated acetone-formaldehyde (SAF) as the dopant to enhance the PSCs stability (
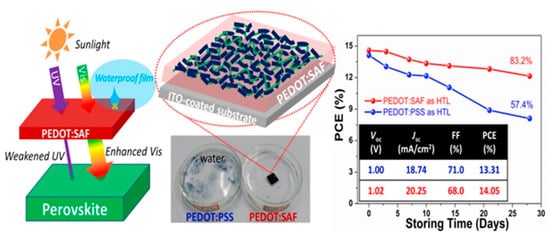
Figure 3) [
56]. PEDOT:SAF exhibited extremely reduced acidity with pH value at around 6 and significantly improved conductivity of 3.12 S/cm. The PEDOT:SAF-based PSC was highly stable with 83.2% of its initial PCE after 28 days of storage. The outstanding characteristics of PEDOT:SAF especially the excellent waterproofness and UV-absorptivity were considered as the key factors for the enhanced device stability.
Figure 3. Schematic diagram showing the reduced UV radiation on pervoskite layer due to absorbtion by the PEDOT:SAF-based HTL. Schematic of proposed structures inside PEDOT:SAF film on ITO-coated substrate. Stability of the PSCs using PEDOT:SAF and PEDOT:PSS as the HTLs. Reprinted with permission from ref. [
56]. Copyright 2017 Elsevier.
Some groups focus on new fabricating methods of PEDOT:PSS film to improve its physical and electrical properties. Erazo et al. deposited PEDOT:PSS layers by an alternative electrochemical (EC) route that offers precise synthesis control, scale-up potential and enhanced cell stability [
104]. The EC-PEDOT:PSS significantly improved the stability of the cells, allowing the devices to maintain 90% of their average efficiency after 15 days. The improved stability is probably related to the lower acidic PSS content in the EC-PEDOT:PSS films. The electrodeposited films present a more hydrophobic nature. Chen et al. proposed a facile strategy of cryo-controlled quasi-congealing spin-coating to improve the PEDOT:PSS quality to a new extent [
105]. This smooth and passivated PEDOT:PSS film facilitates the growth of highly crystalline perovskite and the achievement of better interfacial contact between them, which not only enhance the device efficiency, but also greatly improve the mechanical stability of PSCs under repeated deformation.
Recently, some studies have been reported on incorporating two-dimensional (2D) materials into PSCs to improve the device performance and stability [
40,
110,
111,
112]. 2D materials, such as graphene and its derivatives, black phosphorus, and transition-metal dichalcogenides, with unique van der Waals structure and properties, can increase perovskite film quality and reduce defect states, resulting in better PCE and stronger stability. Therefore, 2D materials have the potential to be incorporated into PEDOT:PSS/PSCs, which could be an interesting research direction for researchers to explore in the future.
This entry is adapted from the peer-reviewed paper 10.3390/nano11113119