Kidney transplantation (KT) is the gold standard treatment of end-stage renal disease. Among the many peri-operative complications that can jeopardize transplant outcomes, ischemia–reperfusion injury (IRI) deserves special consideration as it is associated with delayed graft function, acute rejection, and premature transplant loss. Adipose stem/stromal cells (ASCs) possess specific characteristics that could help prevent, reduce, or reverse IRI.
- adipose stem cells
- extra-cellular vesicles
- kidney transplantation
- ischemia–reperfusion injury
- tolerance
- rejection
- acute kidney injury
- regenerative medicine
1. Introduction
2. Mechanisms of Action
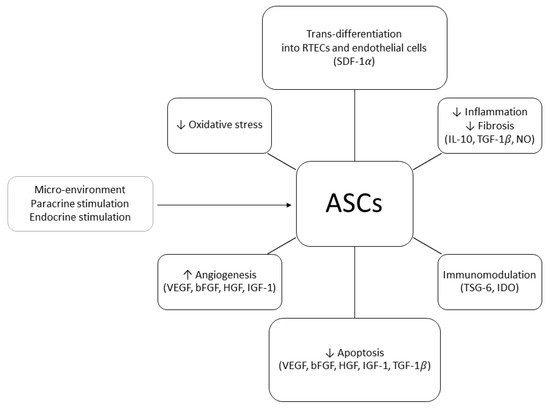
In order to explain the positive effects of ASCs on renal injury, two mechanisms of action have been proposed: differentiative and paracrine. In 2010, Li et al. demonstrated that, in a murine model of acute kidney injury (AKI), human ASCs (hASCs) injected in the tail vein 24 h after the induction of IRI could trans-differentiate into RTECs and repair the damage associated with ATN. At six months of follow-up, the animals treated with hASCs showed normal renal histology whilst the control group exhibited fibrosis and glomerular sclerosis [8]. ASCs’ ability to trans-differentiate into RTECs was confirmed by other studies in which mouse or rabbit kidney scaffolds were seeded with ASCs through the renal artery or ureter [9]. During these experiments, it was observed that ASCs were able to engraft the glomerular, tubular, and vascular areas of the scaffold, eventually trans-differentiating into RTECs and endothelial cells. The authors postulated that the attachment to the scaffold was mediated by the stromal cell-derived factor 1α (SDF-1α
3. Harvesting Procedures
ASCs reside in peri-vascular niches within the adipose tissue, constituting about 20% of the cellular population of the SVF. Two main ASCs harvesting techniques are currently available: lipoaspirate and adipose tissue biopsy. Following specimen collection, the ECM of the adipose tissue undergoes enzymatic or mechanical disruption. The SVF released during the procedure is seeded and expanded in vitro to obtain ASCs [32]. Cultivation and expansion allow to produce a large number of ASCs from a relatively small sample of adipose tissue and permit to collect purified cellular products [33]. However, these methods are expensive, time-consuming, and require specific Good Manufacturing Practice (GMP) facilities. Therefore, it is unlikely that they can be used in the peri-operative setting of a deceased donor KT [34]. Allogenic non-donor derived ASCs represent an intriguing option as off-the-shelf cellular products. Nonetheless, some authors remain concerned that prolonged culture may cause cellular senescence and favor the acquisition of a pro-inflammatory phenotype [35].
4. Timing, Dose, and Route of Administration
This entry is adapted from the peer-reviewed paper 10.3390/ijms222011188
References
- Süsal, C.; Mueller, T.F.; Legendre, C.; Schemmer, P. Editorial: Transplantation of Marginal Organs—Immunological Aspects and Therapeutic Perspectives. Front. Immunol. 2020, 11, 2820.
- Favi, E.; James, A.; Puliatti, C.; Whatling, P.; Ferraresso, M.; Rui, C.; Cacciola, R. Utility and safety of early allograft biopsy in adult deceased donor kidney transplant recipients. Clin. Exp. Nephrol. 2020, 24, 356–368.
- Favi, E.; Puliatti, C.; Iesari, S.; Monaco, A.; Ferraresso, M.; Cacciola, R. Impact of Donor Age on Clinical Outcomes of Primary Single Kidney Transplantation From Maastricht Category-III Donors After Circulatory Death. Transplant. Direct 2018, 4, e396.
- Mallon, D.H.; Summers, D.M.; Bradley, J.A.; Pettigrew, G.J. Defining delayed graft function after renal transplantation: Simplest is best. Transplantation 2013, 96, 885–889.
- Summers, D.M.; Watson, C.J.E.; Pettigrew, G.J.; Johnson, R.J.; Collett, D.; Neuberger, J.M.; Bradley, J.A. Kidney donation after circulatory death (DCD): State of the art. Kidney Int. 2015, 88, 241–249.
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253.
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42.
- Li, K.; Han, Q.; Yan, X.; Liao, L.; Zhao, R.C. Not a process of simple vicariousness, the differentiation of human adipose-derived mesenchymal stem cells to renal tubular epithelial cells plays an important role in acute kidney injury repairing. Stem Cells Dev. 2009, 19, 1267–1275.
- Xue, A.; Niu, G.; Chen, Y.; Li, K.; Xiao, Z.; Luan, Y.; Sun, C.; Xie, X.; Zhang, D.; Du, X.; et al. Recellularization of well-preserved decellularized kidney scaffold using adipose tissue-derived stem cells. J. Biomed. Mater. Res. Part A 2018, 106, 805–814.
- Sabetkish, S.; Sabektish, N.; Ekhtiari, M.; Jobani, B.M.; Kajbafzadeh, A.M. Decellularization and Recellularization of Rabbit Kidney Using Adipose-Derived Mesenchymal Stem Cells for Renal Tissue Engineering. Regen. Eng. Transl. Med. 2020, 6, 433–441.
- Liu, D.; Cheng, F.; Pan, S.; Liu, Z. Stem cells: A potential treatment option for kidney diseases. Stem Cell Res. Ther. 2020, 11, 249.
- Zhou, L.; Song, Q.; Shen, J.; Xu, L.; Xu, Z.; Wu, R.; Ge, Y.; Zhu, J.; Wu, J.; Dou, Q.; et al. Comparison of human adipose stromal vascular fraction and adipose-derived mesenchymal stem cells for the attenuation of acute renal ischemia/reperfusion injury. Sci. Rep. 2017, 7, 1–9.
- Pool, M.B.F.; Vos, J.; Eijken, M.; van Pel, M.; Reinders, M.E.J.; Ploeg, R.J.; Hoogduijn, M.J.; Jespersen, B.; Leuvenink, H.G.D.; Moers, C. Treating Ischemically Damaged Porcine Kidneys with Human Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stromal Cells During Ex Vivo Normothermic Machine Perfusion. Stem Cells Dev. 2020, 29, 1320–1330.
- Engela, A.U.; Baan, C.C.; Peeters, A.M.A.; Weimar, W.; Hoogduijn, M.J. Interaction between adipose tissue-derived mesenchymal stem cells and regulatory T-cells. Cell Transplant. 2013, 22, 41–54.
- Kato, T.; Okumi, M.; Tanemura, M.; Yazawa, K.; Kakuta, Y.; Yamanaka, K.; Tsutahara, K.; Doki, Y.; Mori, M.; Takahara, S.; et al. Adipose tissue-derived stem cells suppress acute cellular rejection by TSG-6 and CD44 interaction in rat kidney transplantation. Transplantation 2014, 98, 277–284.
- Lombardo, E.; DelaRosa, O.; Mancheño-Corvo, P.; Menta, R.; Ramírez, C.; Büscher, D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Eng. Part A 2009, 15, 1579–1589.
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010, 90, 1312–1320.
- Engela, A.U.; Hoogduijn, M.J.; Boer, K.; Litjens, N.H.R.; Betjes, M.G.H.; Weimar, W.; Baan, C.C. Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin. Exp. Immunol. 2013, 173, 343–354.
- Aggarwal, S.; Moggio, A.; Bussolati, B. Concise review: Stem/progenitor cells for renal tissue repair: Current knowledge and perspectives. Stem Cells Transl. Med. 2013, 2, 1011–1019.
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial Progenitor Cell-Derived Extracellular Vesicles: Potential Therapeutic Application in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 6375.
- Wong, D.E.; Banyard, D.A.; Santos, P.J.F.; Sayadi, L.R.; Evans, G.R.D.; Widgerow, A.D. Adipose-derived stem cell extracellular vesicles: A systematic review✰. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1207–1218.
- Collino, F.; Lopes, J.A.; Corrêa, S.; Abdelhay, E.; Takiya, C.M.; Wendt, C.H.C.; De Miranda, K.R.; Vieyra, A.; Lindoso, R.S. Adipose-derived mesenchymal stromal cells under hypoxia: Changes in extracellular vesicles secretion and improvement of renal recovery after ischemic injury. Cell. Physiol. Biochem. 2019, 52, 1463–1483.
- Eirin, A.; Ferguson, C.M.; Zhu, X.-Y.; Saadiq, I.M.; Tang, H.; Lerman, A.; Lerman, L.O. Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney. Stem Cell Res. 2020, 47, 101877.
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067.
- Nargesi, A.A.; Lerman, L.O.; Eirin, A. Mesenchymal Stem Cell-derived Extracellular Vesicles for Renal Repair. Curr. Gene Ther. 2017, 17, 29–42.
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.-N. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538.
- Eirin, A.; Riester, S.M.; Zhu, X.-Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64.
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020, 255, 117719.
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056.
- Liu, C.; Wang, J.; Hu, J.; Fu, B.; Mao, Z.; Zhang, H.; Cai, G.; Chen, X.; Sun, X. Extracellular vesicles for acute kidney injury in preclinical rodent models: A meta-analysis. Stem Cell Res. Ther. 2020, 11.
- Zhang, G.; Wang, D.; Miao, S.; Zou, X.; Liu, G.; Zhu, Y. Extracellular vesicles derived from mesenchymal stromal cells may possess increased therapeutic potential for acute kidney injury compared with conditioned medium in rodent models: A meta-analysis. Exp. Ther. Med. 2016, 11, 1519–1525.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228.
- Storti, G.; Scioli, M.G.; Kim, B.S.; Orlandi, A.; Cervelli, V.; De Francesco, F. Adipose-Derived Stem Cells in Bone Tissue Engineering: Useful Tools with New Applications. Stem Cells Int. 2019, 2019.
- Stivers, K.B.; Beare, J.E.; Chilton, P.M.; Williams, S.K.; Kaufman, C.L.; Hoying, J.B. Adipose-derived cellular erapies in solid organ and vascularized-composite allotransplantation. Curr. Opin. Organ Transplant. 2017, 22, 490–498.
- Eckel-Mahan, K.; Ribas Latre, A.; Kolonin, M.G. Adipose Stromal Cell Expansion and Exhaustion: Mechanisms and Consequences. Cells 2020, 9, 863.
- van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: A systematic review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274.
- Onodera, T.; Fukuhara, A.; Jang, M.H.; Shin, J.; Aoi, K.; Kikuta, J.; Otsuki, M.; Ishii, M.; Shimomura, I. Adipose tissue macrophages induce PPARγ-high FOXP3(+) regulatory T cells. Sci. Rep. 2015, 5, 16801.
- Lynch, L.; Michelet, X.; Zhang, S.; Brennan, P.J.; Moseman, A.; Lester, C.; Besra, G.; Vomhof-Dekrey, E.E.; Tighe, M.; Koay, H.-F.; et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat. Immunol. 2015, 16, 85–95.
- Nishimura, S.; Manabe, I.; Takaki, S.; Nagasaki, M.; Otsu, M.; Yamashita, H.; Sugita, J.; Yoshimura, K.; Eto, K.; Komuro, I.; et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013, 18, 759–766.
- Le Blanc, K.; Davies, L.C. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 168, 140–146.
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51.
- Roemeling-van Rhijn, M.; Reinders, M.E.; Franquesa, M.; Engela, A.U.; Korevaar, S.S.; Roelofs, H.; Genever, P.G.; Ijzermans, J.N.; Betjes, M.G.; Baan, C.C.; et al. Human Allogeneic Bone Marrow and Adipose Tissue Derived Mesenchymal Stromal Cells Induce CD8+ Cytotoxic T Cell Reactivity. J. Stem Cell Res. Ther. 2013, 3, 4.
- Crop, M.J.; Korevaar, S.S.; de Kuiper, R.; IJzermans, J.N.M.; van Besouw, N.M.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Human mesenchymal stem cells are susceptible to lysis by CD8(+) T cells and NK cells. Cell Transplant. 2011, 20, 1547–1559.
- Vanikar, A.V.; Trivedi, H.L.; Thakkar, U.G. Six years’ experience of tolerance induction in renal transplantation using stem cell therapy. Clin. Immunol. 2018, 187, 10–14.
- Ramirez-Bajo, M.J.; Rovira, J.; Lazo-Rodriguez, M.; Banon-Maneus, E.; Tubita, V.; Moya-Rull, D.; Hierro-Garcia, N.; Ventura-Aguiar, P.; Oppenheimer, F.; Campistol, J.M.; et al. Impact of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Rat Model of Kidney Rejection. Front. Cell Dev. Biol. 2020, 8, 1–14.
- Van Rhijn, M.R.; Reinders, M.E.J.; De Klein, A.; Douben, H.; Korevaar, S.S.; Mensah, F.K.F.; Dor, F.J.M.F.; Ijzermans, J.N.M.; Betjes, M.G.H.; Baan, C.C.; et al. Mesenchymal stem cells derived from adipose tissue are not affected by renal disease. Kidney Int. 2012, 82, 748–758.
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell–derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124.
- Grange, C.; Iampietro, C.; Bussolati, B. Stem cell extracellular vesicles and kidney injury. Stem Cell Investig. 2017, 4.
- Ullah, M.; Liu, D.D.; Rai, S.; Razavi, M.; Choi, J.; Wang, J.; Concepcion, W.; Thakor, A.S. A Novel Approach to Deliver Therapeutic Extracellular Vesicles Directly into the Mouse Kidney via Its Arterial Blood Supply. Cells 2020, 9, 937.
- Wu, Q.; Ji, F.-K.; Wang, J.-H.; Nan, H.; Liu, D.-L. Stromal cell-derived factor 1 promoted migration of adipose-derived stem cells to the wounded area in traumatic rats. Biochem. Biophys. Res. Commun. 2015, 467, 140–145.
- Liu, H.; Liu, S.; Li, Y.; Wang, X.; Xue, W.; Ge, G.; Luo, X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE 2012, 7, e34608.
- Chen, Y.-T.T.; Yang, C.-C.C.; Zhen, Y.-Y.Y.; Wallace, C.G.; Yang, J.-L.L.; Sun, C.-K.K.; Tsai, T.-H.H.; Sheu, J.-J.J.; Chua, S.; Chang, C.-L.; et al. Cyclosporine-assisted adipose-derived mesenchymal stem cell therapy to mitigate acute kidney ischemia–reperfusion injury. Stem Cell Res. Ther. 2013, 4, 62.
- Furuichi, K.; Shintani, H.; Sakai, Y.; Ochiya, T.; Matsushima, K.; Kaneko, S.; Wada, T. Effects of adipose-derived mesenchymal cells on ischemia-reperfusion injury in kidney. Clin. Exp. Nephrol. 2012, 16, 679–689.
- Shih, Y.C.; Lee, P.Y.; Cheng, H.; Tsai, C.H.; Ma, H.; Tarng, D.C. Adipose-derived stem cells exhibit antioxidative and antiapoptotic properties to rescue ischemic acute kidney injury in rats. Plast. Reconstr. Surg. 2013, 132, 940–951.
- Fang, B.; Song, Y.P.; Liao, L.M.; Han, Q.; Zhao, R.C. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006, 38, 389–390.
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586.
- Wang, Y.; He, J.; Pei, X.; Zhao, W. Systematic review and meta-Analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology 2013, 18, 201–208.
- Sierra-Parraga, J.M.; Munk, A.; Andersen, C.; Lohmann, S.; Moers, C.; Baan, C.C.; Ploeg, R.J.; Pool, M.; Keller, A.K.; Møller, B.K.; et al. Mesenchymal stromal cells are retained in the porcine renal cortex independently of their metabolic state after renal intra-arterial infusion. Stem Cells Dev. 2019, 28, 1224–1235.
- Kunter, U.; Rong, S.; Boor, P.; Eitner, F.; Müller-Newen, G.; Djuric, Z.; van Roeyen, C.R.; Konieczny, A.; Ostendorf, T.; Villa, L.; et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J. Am. Soc. Nephrol. 2007, 18, 1754–1764.
- Gao, J.; Liu, R.; Wu, J.; Liu, Z.; Li, J.; Zhou, J.; Hao, T.; Wang, Y.; Du, Z.; Duan, C.; et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 2012, 33, 3673–3681.
- Zhou, C.; Zhou, L.; Liu, J.; Xu, L.; Xu, Z.; Chen, Z.; Ge, Y.; Zhao, F.; Wu, R.; Wang, X.; et al. Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomater. 2020, 115, 250–263.
- Zhao, X.; Qiu, X.; Zhang, Y.; Zhang, S.; Gu, X.; Guo, H. Three-Dimensional Aggregates Enhance the Therapeutic Effects of Adipose Mesenchymal Stem Cells for Ischemia-Reperfusion Induced Kidney Injury in Rats. Stem Cells Int. 2016, 2016.
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213.
- Ferraresso, M.; Favi, E. Hypothermic Machine Perfusion in Kidney Transplantation: Back to the Future? J. Transplant. Technol. Res. 2015, 6.
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19.
- Iwai, S.; Sakonju, I.; Okano, S.; Teratani, T.; Kasahara, N.; Yokote, S.; Yokoo, T.; Kobayash, E. Impact of ex vivo administration of mesenchymal stem cells on the function of kidney grafts from cardiac death donors in rat. Transplant. Proc. 2014, 46, 1578–1584.
- Wang, Y.L.; Li, G.; Zou, X.F.; Chen, X.B.; Liu, T.; Shen, Z.Y. Effect of autologous adipose-derived stem cells in renal cold ischemia and reperfusion injury. Transplant. Proc. 2013, 45, 3198–3202.
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J. Cell. Mol. Med. 2017, 21, 3381–3393.
- Pool, M.; Eertman, T.; Parraga, J.S.; ’t Hart, N.; van Rhijn, M.R.; Eijken, M.; Jespersen, B.; Reinders, M.; Hoogduijn, M.; Ploeg, R.; et al. Infusing mesenchymal stromal cells into porcine kidneys during normothermic machine perfusion: Intact MSCs can be traced and localised to Glomeruli. Int. J. Mol. Sci. 2019, 20, 3607.
