Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Essential oils from different plant species were found to contain different compounds exhibiting anti-inflammatory effects with the potential to be a valid alternative to conventional chemotherapy that is limited in long-term use due to its serious side effects.
- inflammation
- oils
1. Plant-Derived Essential Oils
An essential oil (EO) is generally defined as a product obtained from a natural raw material of plant origin, usually by steam distillation or by mechanical processes [1]. These plant products are very complex natural mixtures of secondary volatile metabolites produced by aromatic plants, where they represent chemical defences against herbivores and pathogens such as bacteria, viruses and fungi [2]. They also exert a dual role in attracting pollinating insects and in repelling the harmful ones [3]. In plants, essential oils are synthesized by all tissues and are stored in secretory cells, epidermic cells or glandular trichomes; consequently, they can be extracted from any plant organs such as buds, stems, twigs, leaves, roots, wood, bark, flowers, fruits and seeds [3]. The particular composition of each EO depends not only on the plant species or plant tissue from which it is extracted but also on the climate, on the soil composition, on the vegetative cycle stage or age and even on the time of the harvest [4]. Moreover, the chemical profile is also affected by the extraction method carried out, thus highlighting the importance of specific extraction techniques over the steam distillation, such as solvent extraction, Soxhlet extraction, microwave-assisted hydro distillation, solvent flavour evaporation, etc., in compliance with the plant material characteristics [5]. Their peculiar chemical composition is mainly represented by various terpenoids and their oxygenated derivatives, along with aldehydes and ketones, esters and alcohols [4]. Generally, they are colourless volatile liquids soluble in organic solvents with a density lower than that of water. The most commercial EOs are extracted from various aromatic plants growing in temperate and warm regions of the Mediterranean and tropical areas, where they are historically used in traditional medicine against a wide variety of pathological conditions due to their numerous pharmacological activities including antimicrobial, antiviral, antioxidant and anti-inflammatory effects [3].
Chronic inflammation and oxidative stress are associated with most of the common chronic disorders and diseases [6][7]. It is known that the normal functions of biological molecules (such as proteins, lipids and DNA) are destabilized by oxidative stress sustained by free radicals (ROS, NRS), which also affects many inflammation-related signalling pathways, thus influencing the cellular and tissues homeostasis. On the other hand, chronic inflammation is characterized by the production of proinflammatory cytokines and chemokines, which leads to pain, redness and swelling of the involved tissue [8]. In traditional medicine, EOs have been used for the treatment of inflammatory processes [9], as they possess many beneficial properties due to the presence of several antioxidant and anti-inflammation compounds such as terpenes, the main class of compounds, and especially monoterpenes [5]. They are also present in numerous pharmaceutical products [10]. In recent studies, Citrus bergamia Risso and Poiteau juice (known as Bergamot) on cardiometabolic risk in dyslipidemic subjects was shown to significantly reduce plasma lipids and improve the atherogenic lipoproteins and subclinical atherosclerosis [11]. Other recent studies with chlorogenic acid and luteolin-based supplement from artichoke extract showed an improvement of two early atherosclerotic markers, carotid intima-media thickness and flow-mediated dilation, evidencing a clinical relevance, considering their beneficial nutraceutical properties, on vascular function and remodelling, including a beneficial cardiovascular and hepatoprotective effects [12].
2. Essential Oils from Lamiaceae Family
2.1. Lavandula x intermedia (Lavender)
The lavender EOs are generally composed mainly by camphor, terpinen-4-ol, linalool, linalyl acetate, beta-ocimene and 1,8-cineole [13]. Immunomodulatory and anti-inflammatory properties of compounds found in the lavender EOs have also been reported [14]. Many inflammatory processes are associated with leukotriene production catalysed by lipoxygenase (LOX), which can use molecular oxygen or hydrogen peroxide as oxidants. L. x intermedia EOs showed a moderate antioxidant activity, mainly attributed to the effect of linalool and linalyl acetate. These findings supported the use of EOs of L. X intermedia as natural ingredients useful for gastrointestinal disorders and for oxidative stress-related diseases [15].
2.2. Rosmarinus officinalis (Rosemary)
Characteristic EOs of Rosmarinus officinalis include 1,8-cineole, α-pinene, camphor, bornyl acetate, borneol, camphene, α-terpineol, limonene, β-pinene, β-caryophyllene and myrcene. In traditional medicine, it is used for the treatment of inflammation-related disorders [16]. Yet, this plant exerts antioxidant activity and prevents inflammatory ROS-related injury, stimulates smooth muscle relaxation and has low toxicity. Bustanji et al. performed a study to identify the effects of rosemary in blood glucose and lipid profile. They reported through an in vitro assay that rosemary extract (with the component gallic acid: IC50 14.5 μg/mL) inhibited in a dose-dependent manner the activity of cyclic adenosine monophosphate of gluconeogenic genes, cytosolic phosphoenol-pyruvate carboxykinase and glucose-6-phosphatase. Rosemary extract showed hypoglycaemic and hypolipidemic effects by activation of signalling pathways including AMP-activated protein kinase (which induces glycolysis) and proliferator-activated receptor gamma (PPAR-γ). It upregulated the expression of LDL-C receptor (responsible of the endocytosis of LDL-C from blood plasma to liver hepatocytes), sirtuin-1 (increasing the oxidation of fatty acids) and PGC1α (activates PPAR-γ) [17]. Neutrophils are rapidly mobilized, and they are one of the first and main cells to arrive at the inflammation site. Oral treatment with R. officinalis aqueous extract reduced the neutrophil influx, the release of cytokines and the oxidative stress on inflamed exudates [18]. The study of Borges et al. [19] showed that all nanoemulsions showed no toxicity and also showed the ability to potentiate the anti-inflammatory action of essential oils by exerting immunomodulatory activity by inhibiting the production of the proinflammatory mediator nitric oxide.
Plant extracts and their compounds have proven to be an alternative for treating periodontal diseases, since rosemary has shown antibacterial and anti-inflammatory activities in toothpaste presentation, which reduced biofilm formation and improved gingival bleeding [20]. Rasooli et al. also reported an in vivo reduction of biofilm by rosemary EO and suggested its potential use as an anticaries agent [21]. Bernardes et al. also confirmed this antimicrobial activity against oral bacteria in their study. These authors used common bacterial species from the oral cavity (Streptococcus mutans, Streptococcus mitis, Streptococcus sanguinis, Streptococcus salivarius, Streptococcus sobrinus and Enterococcus faecalis) in planktonic form, and the greatest antimicrobial activity of rosemary EO was shown against Streptococcus mitis [22]. The results of Smullen et al. have shown that rosemary and other plant-derived extracts inhibited growth and adhesion of oral bacteria to glass, inhibited both glucosyltransferase activity and glucan production by S. mutans and prevented plaque formation in vitro and on bovine teeth [23].
2.3. Thymus capitatus (Thyme)
The main single constituents of EOs from Thymus genus are thymol, carvacrol, linalool, a-terpineol, 1,8-cineole and borneol [24]. Iauk et al. [25] have investigated the hypoglycaemic activity of T. capitatus (L.) Hoffsgg. & Link via the inhibition of α-amylase and α-glucosidase, inhibitors that offer an attractive strategy to control postprandial hyperglycaemia for type 2 diabetes management. Manconi et al. [26] had suggested that formulations on Thymus capitatus EO in phospholipid vesicles might be used as an antibacterial–antioxidant mouthwash for the treatment of oral cavity diseases. Finally, Valerio et al. [27] indicated the potential use of thyme as biopreservative for bakery products due to its antimicrobial properties. The antioxidant activity of the formulations was evaluated as a protector of keratinocytes against the damage induced by hydrogen peroxide. They were capable of favouring wound repair in keratinocytes. The antibacterial activity of the EO was demonstrated against cariogenic Streptococcus mutans, Lactobacillus acidophilus and commensal Streptococcus sanguinis [26]. Alvarez-Echazú et al. used thymol–chitosan hydrogels to protect the dental biofilm from breakdown and treat inflammation [28]. Thymus zygis has been studied more extensively from an immunological point of view. In a cellular model with human macrophages the gene expression for IL-1β, TNFα and Il-6 were significantly reduced, and anti-inflammatory cytokines such as IL-10 dose-dependently highly increased [29]. Thymus zygis has also been tested in vitro as an antibacterial agent against E. coli, S. enteritidis, S. essen and other bacterial species [30]. Interestingly, in addition to antioxidant, anti-inflammatory, antimicrobial, spasmolytic, antinociceptive and antitumor activity, some EOs, such as those from Thymus vulgaris L. may enhances cognitive function, as shown in animal models [31].
2.4. Selected Compounds as Essential Oils from Lamiaceae Family
The following table presents a summary of the main compounds of EOs from the Lamiaceae family and their effects on inflammatory processes.
Terpineols are isomers of monocyclic monoterpene alcohol naturally present in different plants, among which the most common are α-terpineol and terpinen-4-ol [32] (Figure 1). In particular, the latter has been shown in vitro to suppress inflammatory mediator production by activation of monocytes [33] and to inhibit inflammatory cytokine generation in LPS-stimulated human macrophages [34] but also in animal models to attenuate inflammation in dextran sulphate sodium-induced colitis [35], prevent LPS-induced acute lung injury by decreasing LPS-induced NF-κB activation and trigger peroxisome PPAR-γ [36] (Figure 2).
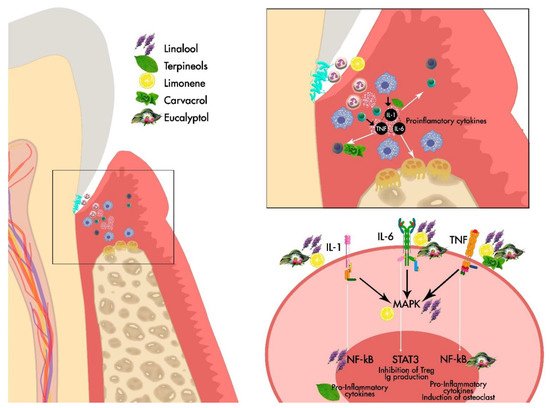
Figure 1. General Scheme of the effect of the main EOs derived from Lamiaceae family on the periodontium.
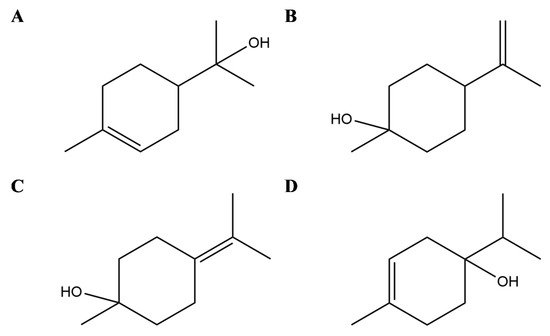
Figure 2. Selected compounds from Essential Oils from Lamiaceae family. Terpineols: α- terpineol (A), β- terpineol (B), γ-terpineol (C), and terpinen-4-ol (D).
Linalool (3,7-dimethyl-1,6-octadien-3-ol) is an acyclic monoterpene found in EOs of hundreds of plants widely spread worldwide and principally in Lamiaceae family [37] (Figure 3A). Several in vitro and in vivo studies demonstrated different anti-inflammatory effects of this monoterpene also interfering with the mediators of the inflammation pathways. In detail, in RAW 264.6 monocyte/macrophage-like cells linalool decreased the generation of lipopolysaccharide (LPS)-induced TNF-α and IL-6 and inhibited the activation of the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways [38]. In addition, in animal models, it has been shown that linalool attenuated acute lung inflammation by reducing TNF-α, IL-6, IL-8, IL-1β and monocyte chemoattractant protein-1 (MCP-1) production [39], further supporting linalool as a promising tool to treat inflammatory related diseases.
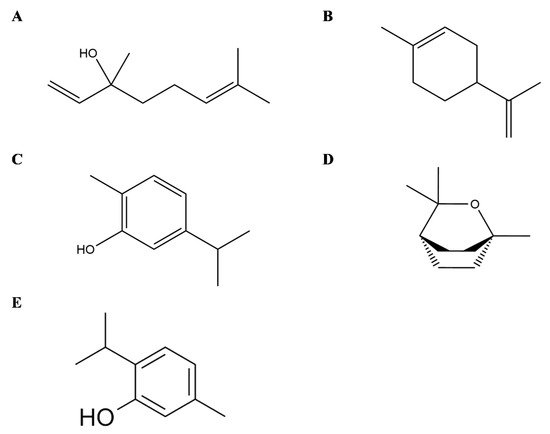
Figure 3. Selected compounds from Essential Oils from Lamiaceae family. Linalool (A), Limonene (B), Carvacrol (C), Eucalyptol (D), Thymol (E).
Limonene (1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene) is a cyclic monoterpene and one of the most common terpenes in nature as well as the main constituent of citrus Eos (Figure 3B). Its anti-inflammatory effects are principally linked to the modulation of cytokines and the interference with the inflammatory-related pathways, as demonstrated by in vitro and in vivo assays. Limonene decreases leukocytes infiltration and neutrophils migration, as well as the levels of TNF-α in cell derived from the peritoneal cavity and in the peritoneal exudate of zymosan-induced peritonitis BALB/C mice [40]. In LPS inflammation-induced RAW 264.7 macrophages, limonene reduced in a dose-dependent manner the levels of proinflammatory cytokines TNF-α, IL-6 and IL-1β, together with the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX) and prostaglandin E2 (PGE2) [41]. Similarly, in vitro model of osteoarthritis with IL-1β-stimulated human chondrocytes, limonene negatively modulated nitric oxide (NO) production by decreasing iNOS, matrix metalloproteinase (MMP)-1 and MMP-13expression, besides NF-κB and p38 activation [42].
Carvacrol (5-isopropyl-2-methylphenol) is a cyclic monoterpene mainly present in the EO of plants from Lamiaceae family (Figure 3C). In an experimental rat model of periodontal disease, carvacrol maintained alveolar bone resorption and decreased tissue lesion at histopathology, with preservation of the gingival tissue also demonstrating anti-inflammatory and antibacterial activities [43]. In addition, in vitro in murine macrophages carvacrol (1, 10, and 100 μg/mL) reduced the LPS-induced nitrite production as well as in vivo in a model of carrageenan-induced pleurisy with a pretreatment 50 or 100 mg/kg, i.e., carvacrol reduced the levels of TNF-α and suppressed leukocytes recruitment in pleural lavage [44]. In human macrophage-like U937 cells, carvacrol suppressed LPS-induced COX-2 expression, activating PPARγ, indicating its anti-inflammatory properties [45] and having no selectivity for both COX-1 and COX-2 enzyme isoforms [46]. Furthermore, it has been shown that carvacrol induces Nav blockade in DRG neurons [47][48], and Gonçalves et al. also reported significant analgesic activity and dose-dependency of T. capitatus EO. It is suggest that it is the main active molecule behind the antinociceptive effects of T. capitatus through peripheral nervous excitability blockade [49]. Other study showed that carvacrol and thymol have the most potent antimicrobial activity against Escherichia coli, Sta. aureus, Str. epidermidis, Enterococcus faecalis, Yersinia enterocolitica, Candida albicans, Bacillus cereus, Listeria monocytogenes, Salmonella typhimurium and Saccharomyces cerevisiae, with the exception of Pseudomonas aeruginosa [50]. Taking into account that T. capitatus fractions are characterized by the presence of carvacrol as dominant constituent, the antibacterial properties may be attributed to this oxygenated monoterpene.
Thymol (2-isopropyl-5-methylphenol), a monoterpene phenol, is a typical compound in EOs from thyme species (Figure 3E). Thymol also ameliorates inflammation in vitro in LPS-induced inflammation in murine macrophage cells [51] as well as in LPS- and interferon (IFN)-γ-induced macrophage inflammation, besides inhibition of the iNO RNA expression in J774A.1 cells [52]. Thymol may also modify prostaglandin catalysed biosynthesis by the inhibition of COX-1 and COX-2 isoforms [53]. Moreover, in mouse mammary epithelial cells, LPS-induced inflammatory response was decreased after thymol treatment (40 μg/mL) by the downregulation of MAPK and NF-κB signalling pathways [54]. Recently, Perrino et al. [55] have reported a high bioactivity also found in endemic wild species as spinulosus Ten., indicating its potential use in organic agriculture, since thymol may also serve as natural agent against phytopathogenic microorganisms.
Eucalyptol or 1,8-cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane) is a bicyclic monoterpene isolated from EOs from numerous plants [56] (Figure 3D). Its anti-inflammatory properties have also been investigated in human and animal models of respiratory diseases such as asthma, Chronic Obstructive Pulmonary Disease and bronchitis [57][58][59]. In vitro studies on LPS-induced human lymphocytes and monocytes showed a reduced expression of cytokines including TNFα, IL-6 and IL-1β accompanied with a decrease in NFκB activated form [60][61].
This entry is adapted from the peer-reviewed paper 10.3390/app11209563
References
- Ríos, J.-L. Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 3–10.
- Ul Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739.
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70.
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21.
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110.
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80.
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214.
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130.
- Bonesi, M.; Loizzo, M.R.; Acquaviva, R.; Malfa, G.A.; Aiello, F.; Tundis, R. Anti-inflammatory and Antioxidant Agents from Salvia Genus (Lamiaceae): An Assessment of the Current State of Knowledge. Antiinflamm. Antiallergy Agents Med. Chem. 2017, 16, 70–86.
- Dajic Stevanovic, Z.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural Macromolecules as Carriers for Essential Oils: From Extraction to Biomedical Application. Front. Bioeng. Biotechnol. 2020, 8, 563.
- Toth, P.P.; Patti, A.M.; Nikolic, D.; Giglio, R.V.; Castellino, G.; Biancucci, T.; Geraci, F.; David, S.; Montalto, G.; Rizvi, A.; et al. Bergamot Reduces Plasma Lipids, Atherogenic Small Dense LDL, and Subclinical Atherosclerosis in Subjects with Moderate Hypercholesterolemia: A 6 Months Prospective Study. Front. Pharmacol. 2015, 6, 299.
- Castellino, G.; Nikolic, D.; Magan-Fernandez, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix((R)) Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580.
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308.
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Cienc. 2015, 87, 1397–1408.
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandin (Lavandula x intermedia Emeric ex Loiseleur) essential oil from Spain: Determination of aromatic profile by gas chromatography-mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Nat. Prod. Res. 2016, 30, 1123–1130.
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283.
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Almasri, I.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2010, 4, 2235–2242.
- Silva, A.M.; Machado, I.D.; Santin, J.R.; de Melo, I.L.; Pedrosa, G.V.; Genovese, M.I.; Farsky, S.H.; Mancini-Filho, J. Aqueous extract of Rosmarinus officinalis L. inhibits neutrophil influx and cytokine secretion. Phytother. Res. 2015, 29, 125–133.
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; Dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceicao, E.C.; et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: In vitro and in zebrafish studies. Inflammopharmacology 2018, 26, 1057–1080.
- Valones, M.A.A.; Silva, I.C.G.; Gueiros, L.A.M.; Leao, J.C.; Caldas, A.F., Jr.; Carvalho, A.A.T. Clinical Assessment of Rosemary-based Toothpaste (Rosmarinus officinalis Linn.): A Randomized Controlled Double-blind Study. Braz. Dent. J. 2019, 30, 146–151.
- Rasooli, I.; Shayegh, S.; Taghizadeh, M.; Astaneh, S.D. Phytotherapeutic prevention of dental biofilm formation. Phytother. Res. 2008, 22, 1162–1167.
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Flauzino, L.G.; Souza, M.G.; Turatti, I.C.; Andrade e Silva, M.L.; Martins, C.H.; da Silva Filho, A.A.; Cunha, W.R. Antibacterial activity of the essential oil from Rosmarinus officinalis and its major components against oral pathogens. Z. Nat. C 2010, 65, 588–593.
- Smullen, J.; Finney, M.; Storey, D.M.; Foster, H.A. Prevention of artificial dental plaque formation in vitro by plant extracts. J. Appl. Microbiol. 2012, 113, 964–973.
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706.
- Iauk, L.; Acquaviva, R.; Mastrojeni, S.; Amodeo, A.; Pugliese, M.; Ragusa, M.; Loizzo, M.R.; Menichini, F.; Tundis, R. Antibacterial, antioxidant and hypoglycaemic effects of Thymus capitatus (L.) Hoffmanns. et Link leaves’ fractions. J. Enzyme Inhib. Med. Chem. 2015, 30, 360–365.
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122.
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and Antimicrobial Properties of Essential Oils from Four Wild Taxa of Lamiaceae Family Growing in Apulia. Agronomy 2021, 11, 1431.
- Alvarez Echazu, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 588–596.
- Ocana, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on Cytokine Production and Gene Expression of oxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706.
- Penalver, P.; Huerta, B.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS 2005, 113, 1–6.
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris Essential Oil Protects Zebrafish against Cognitive Dysfunction by Regulating Cholinergic and Antioxidants Systems. Antioxidants 2020, 9, 1083.
- Khaleel, C.; Tabanca, N.; Buchbauer, G.J.O.C. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361.
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626.
- Nogueira, M.N.; Aquino, S.G.; Rossa Junior, C.; Spolidorio, D.M. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1beta, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778.
- Zhang, H.; Hua, R.; Zhang, B.; Zhang, X.; Yang, H.; Zhou, X. Serine Alleviates Dextran Sulfate Sodium-Induced Colitis and Regulates the Gut Microbiota in Mice. Front. Microbiol. 2018, 9, 3062.
- Peng, L.Y.; Shi, H.T.; Yuan, M.; Li, J.H.; Song, K.; Huang, J.N.; Yi, P.F.; Shen, H.Q.; Fu, B.D. Madecassoside Protects Against LPS-Induced Acute Lung Injury via Inhibiting TLR4/NF-kappaB Activation and Blood-Air Barrier Permeability. Front. Pharmacol. 2020, 11, 807.
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543.
- Huo, M.; Gao, R.; Jiang, L.; Cui, X.; Duan, L.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Suppression of LPS-induced inflammatory responses by gossypol in RAW 264.7 cells and mouse models. Int. Immunopharmacol. 2013, 15, 442–449.
- Ma, J.; Xu, H.; Wu, J.; Qu, C.; Sun, F.; Xu, S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-kappaB activation. Int. Immunopharmacol. 2015, 29, 708–713.
- Kummer, R.; Fachini-Queiroz, F.C.; Estevao-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K. Evaluation of Anti-Inflammatory Activity of Citrus latifolia Tanaka Essential Oil and Limonene in Experimental Mouse Models. Evid. Based Complement. Alternat. Med. 2013, 2013, 859083.
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421.
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150.
- Botelho, M.A.; Rao, V.S.; Montenegro, D.; Bandeira, M.A.; Fonseca, S.G.; Nogueira, N.A.; Ribeiro, R.A.; Brito, G.A. Effects of a herbal gel containing carvacrol and chalcones on alveolar bone resorption in rats on experimental periodontitis. Phytother. Res. 2008, 22, 442–449.
- Guimaraes, A.G.; Xavier, M.A.; de Santana, M.T.; Camargo, E.A.; Santos, C.A.; Brito, F.A.; Barreto, E.O.; Cavalcanti, S.C.; Antoniolli, A.R.; Oliveira, R.C.; et al. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 253–263.
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J. Lipid Res. 2010, 51, 132–139.
- Landa, P.; Kokoska, L.; Pribylova, M.; Vanek, T.; Marsik, P. In vitro anti-inflammatory activity of carvacrol: Inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch. Pharm. Res. 2009, 32, 75–78.
- Joca, H.C.; Vieira, D.C.; Vasconcelos, A.P.; Araujo, D.A.; Cruz, J.S. Carvacrol modulates voltage-gated sodium channels kinetics in dorsal root ganglia. Eur. J. Pharmacol. 2015, 756, 22–29.
- Joca, H.C.; Cruz-Mendes, Y.; Oliveira-Abreu, K.; Maia-Joca, R.P.; Barbosa, R.; Lemos, T.L.; Lacerda Beirao, P.S.; Leal-Cardoso, J.H. Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J. Nat. Prod. 2012, 75, 1511–1517.
- Goncalves, J.C.; de Meneses, D.A.; de Vasconcelos, A.P.; Piauilino, C.A.; Almeida, F.R.; Napoli, E.M.; Ruberto, G.; de Araujo, D.A. Essential oil composition and antinociceptive activity of Thymus capitatus. Pharm. Biol. 2017, 55, 782–786.
- Cosentino, S.; Tuberoso, C.I.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135.
- Chauhan, A.K.; Jakhar, R.; Paul, S.; Kang, S.C. Potentiation of macrophage activity by thymol through augmenting phagocytosis. Int. Immunopharmacol. 2014, 18, 340–346.
- Vigo, E.; Cepeda, A.; Gualillo, O.; Perez-Fernandez, R. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: Nitric oxide inhibition in J774A.1 murine macrophages. J. Pharm. Pharmacol. 2004, 56, 257–263.
- Marsik, P.; Kokoska, L.; Landa, P.; Nepovim, A.; Soudek, P.; Vanek, T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005, 71, 739–742.
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-kappaB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222.
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952.
- Yalcin, H.; Anik, M.; Sanda, M.A.; Cakir, A. Gas chromatography/mass spectrometry analysis of Laurus nobilis essential oil composition of northern Cyprus. J. Med. Food 2007, 10, 715–719.
- Bastos, V.P.; Gomes, A.S.; Lima, F.J.; Brito, T.S.; Soares, P.M.; Pinho, J.P.; Silva, C.S.; Santos, A.A.; Souza, M.H.; Magalhaes, P.J. Inhaled 1,8-cineole reduces inflammatory parameters in airways of ovalbumin-challenged Guinea pigs. Basic Clin. Pharmacol. Toxicol. 2011, 108, 34–39.
- Kennedy-Feitosa, E.; Okuro, R.T.; Pinho Ribeiro, V.; Lanzetti, M.; Barroso, M.V.; Zin, W.A.; Porto, L.C.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm. Pharmacol. Ther. 2016, 41, 11–18.
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256.
- Juergens, U.R.; Engelen, T.; Racke, K.; Stober, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287.
- Kim, K.Y.; Lee, H.S.; Seol, G.H. Eucalyptol suppresses matrix metalloproteinase-9 expression through an extracellular signal-regulated kinase-dependent nuclear factor-kappa B pathway to exert anti-inflammatory effects in an acute lung inflammation model. J. Pharm. Pharmacol. 2015, 67, 1066–1074.
This entry is offline, you can click here to edit this entry!
