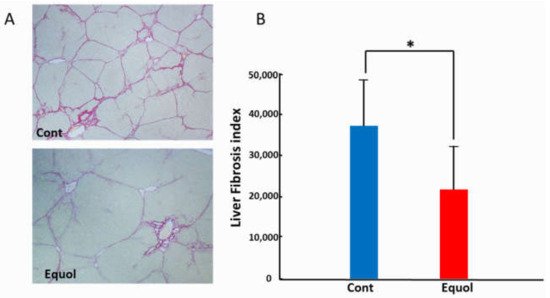
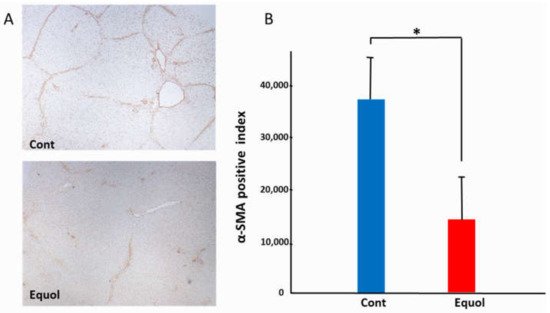
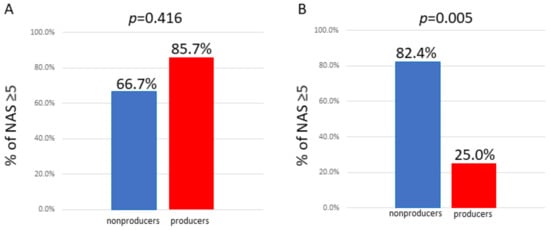
Equol is a metabolite of daidzein, a major soybean isoflavone with estrogenic and antioxidant activities. As the production of equol depends on the presence of certain members of the intestinal microflora, not all individuals can produce equol. Treatment with equol-rich soy product, SE5-OH markedly attenuated the development of liver fibrosis and the expression of alpha-smooth muscle actin in obese OLETF rats, and in the clinical study, the degree of fibrosis and ballooning in equol nonproducers was significantly higher than that of producers in women with NASH. In addition, the percentage of nonproducers with NAS ≥ 5 was significantly higher than that of producers in women with NASH.
- equol
- nonalcoholic steatohepatitis
- estrogen
1. Introduction
2. Animal Study
3. Clinical Research
3.1. Subject Baseline Characteristics
| Variable | Nonproducer (n = 23) | Producer (n = 15) | p-Value |
|---|---|---|---|
| Age (y) | 54.6 ± 15.0 | 60.4 ± 9.3 | 0.697 |
| Sex (Male/Female) | (6/17) | (7/8) | 0.191 |
| Equol (nmol/mL) | BLQ | 23.0 ± 34.2 | |
| Daidzein (nmol/mL) | 50.2 ± 46.1 | 12.0 ± 7.4 | 0.01 |
| Log (Equol/Daidzein) | - | 0.0 ± 0.75 | |
| Hypertension (%) | 11 (47.8) | 7 (46.7) | 0.944 |
| Dyslipidemia (%) | 11 (47.8) | 5 (33.3) | 0.376 |
| Diabetes Mellitus (%) | 11 (47.8) | 12 (80.0) | 0.047 |
| BMI (kg/m2) | 28.6 ± 4.2 | 28.5 ± 4.1 | 0.945 |
| Platelet count (×104/μL) | 20.3 ± 6.4 | 16.4 ± 5.5 | 0.062 |
| AST (IU/L) | 62.0 ± 26.7 | 62.3 ± 35.4 | 0.97 |
| ALT (IU/L) | 78.8 ± 45.4 | 73.0 ± 41.2 | 0.696 |
| Fasting glucose (mg/dL) | 103.6 ± 24.7 | 115.7 ± 30.0 | 0.205 |
| HOMA-IR | 5.8 ± 5.8 | 11.6 ± 11.1 | 0.129 |
| Ferritin | 173.5 ± 192.9 | 238.0 ± 128.0 | 0.278 |
| Type 4 collagen 7S (ng/mL) | 6.2 ± 3.1 | 6.0 ± 2.2 | 0.822 |
| P-III-P (U/mL) | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.895 |
| FIB-4 index | 2.5 ± 1.6 | 3.3 ± 2.7 | 0.258 |
| APRI | 1.1 ± 0.7 | 1.5 ± 1.3 | 0.214 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Variable | Nonproducer (n = 6) |
Producer (n = 7) |
p-Value | Nonproducer (n = 17) |
Producer (n = 8) |
p-Value |
| Age, y | 53.4 ± 17.9 | 56.0 ± 8.4 | 0.734 | 60.5 ± 14.0 | 64.1± 8.8 | 0.512 |
| Equol (nmol/mL) | BLQ | 34.3 ± 46.5 | BLQ | 11.7 ± 8.9 | ||
| Daidzein (nmol/mL) | 52.1 ± 41.7 | 10.7 ± 7.9 | 0.059 | 49.5 ± 48.9 | 13.4 ± 7.1 | 0.011 |
| Log (Equol/Daidzein) | - | 0.17 ± 0.73 | - | −0.2 ± 0.8 | ||
| Hypertension (%) | 3 (50.0) | 4 (57.1) | 0.797 | 8 (47.1) | 3 (37.5) | 0.653 |
| Dyslipidemia (%) | 2 (33.3) | 4 (57.1) | 0.391 | 9 (52.9) | 1 (12.5) | 0.054 |
| Diabetes Mellitus (%) | 4 (66.7) | 5 (71.4) | 0.853 | 7 (41.2) | 7 (87.5) | 0.03 |
| Menopause | - | - | - | 14 (82.4) | 7 (87.5) | 0.743 |
| BMI (kg/m2) | 28.9 ± 2.6 | 28.5 ± 3.7 | 0.852 | 28.5 ± 4.7 | 28.5 ± 4.7 | 0.997 |
| Platelet count (×104/μL) | 19.3 ± 4.9 | 17.6 ± 5.0 | 0.552 | 20.6 ± 6.9 | 15.4 ± 6.0 | 0.078 |
| AST (IU/L) | 60.2 ± 16.3 | 60.0 ± 24.3 | 0.989 | 62.6 ± 30.1 | 64.4 ± 44.7 | 0.907 |
| ALT (IU/L) | 94.5 ± 45.7 | 78.4 ± 45.6 | 0.54 | 73.2 ± 45.3 | 68.3 ± 39.4 | 0.797 |
| Fasting glucose (mg/dL) | 113.3 ± 40.7 | 129.9 ± 27.8 | 0.405 | 100.1 ± 16.5 | 103.3 ± 27.6 | 0.725 |
| HOMA-IR | 9.4 ± 9.8 | 16.7 ± 14.7 | 0.397 | 4.4 ± 2.9 | 8.6 ± 8.2 | 0.101 |
| Ferritin | 273.8 ± 352.4 | 242.6 ± 140.0 | 0.834 | 144.0 ± 117.2 | 233.4 ± 126.5 | 0.111 |
| Type 4 collagen 7S (ng/ML) | 4.8 ± 0.9 | 5.6 ± 2.9 | 0.634 | 6.5 ± 3.3 | 6.3 ± 1.7 | 0.853 |
| P-III-P (U/mL) | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.831 | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.803 |
| FIB-4 index | 1.9 ± 1.0 | 2.5 ± 1.1 | 0.414 | 2.7 ± 1.8 | 4.1 ± 3.5 | 0.317 |
| APRI | 1.1 ± 0.3 | 1.3 ± 0.6 | 0.495 | 1.1 ± 0.7 | 1.7 ± 1.7 | 0.358 |
Values are n (%) or mean ± standard deviation. BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA-IR, homeostasis model assessment-insulin resistance, type III procollagen peptide; P-III-P, the aspartate aminotransferase to platelet ratio index; APRI, below the limit of quantitation; BLQ.
3.2. Comparison of Pathological Features between Equol Nonproducers and Producers
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Nonproducer n, (%) |
Producer n, (%) |
p-Value | Nonproducer n, (%) |
Producer n, (%) |
p-Value | |
| Fibrosis stage | 0.292 | 0.047 | ||||
| 0 | 1, (16.7) | 0, (0) | 2, (11.8) | 0, (0) | ||
| 1 | 1, (16.7) | 0, (0) | 3, (17.6) | 0, (0) | ||
| 2 | 1, (16.7) | 1, (14.3) | 4, (23.5) | 7, (87.5) | ||
| 3 | 3, (50.0) | 3, (42.9) | 4, (23.5) | 1, (12.5) | ||
| 4 | 0, (0) | 3, (42.9) | 4, (23.5) | 0, (0) | ||
| Steatosis | 0.629 | 0.44 | ||||
| 1 | 0, (0) | 1, (14.3) | 6, (35.3) | 5, (62.5) | ||
| 2 | 5, (83.3) | 5, (71.4) | 7, (41.2) | 2, (25.0) | ||
| 3 | 1, (16.7) | 1, (14.3) | 4, (23.5) | 1, (12.5) | ||
| Lobular inflammation | 0.489 | 0.262 | ||||
| 0 | 0, (0) | 0, (0) | 1, (5.9) | 0, (0) | ||
| 1 | 2, (33.3) | 2, (28.6) | 5, (29.4) | 5, (62.5) | ||
| 2 | 3, (50.0) | 5, (71.4) | 11, (64.7) | 3, (37.5) | ||
| 3 | 1, (16.7) | 0, (0) | 0, (0) | 0, (0) | ||
| Ballooning | 0.612 | 0.03 | ||||
| 1 | 5, (83.3) | 5, (71.4) | 7, (41.2) | 7, (87.5) | ||
| 2 | 1, (16.7) | 2, (28.6) | 10, (58.8) | 1, (12.5) | ||
| NAS score | 0.292 | 0.084 | ||||
| 3 | 0, (0) | 1, (14.3) | 2, (11.8) | 3, (37.5) | ||
| 4 | 2, (33.3) | 0, (0) | 1, (5.9) | 3, (37.5) | ||
| 5 | 2, (33.3) | 4, (57.1) | 9, (52.9) | 1, (12.5) | ||
| 6 | 1, (16.7) | 2, (28.6) | 4, (23.5) | 1, (12.5) | ||
| 7 | 1, (16.7) | 0, (0) | 1, (5.9) | 0, (0) | ||
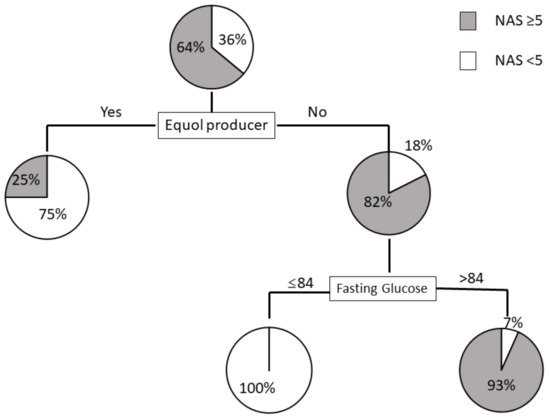
3.3. Prediction Model for NAS ≥ 5 Using Decision Trees Analysis
This entry is adapted from the peer-reviewed paper 10.3390/ijms222111904
References
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682.
- Peng, C.; Stewart, A.G.; Woodman, O.L.; Ritchie, R.H.; Qin, C.X. Non-Alcoholic Steatohepatitis: A Review of Its Mechanism, Models and Medical Treatments. Front. Pharmacol. 2020, 11, 603926.
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. (Lond.) 2008, 32, 949–958.
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414.
- Xu, X.; Harris, K.S.; Wang, H.J.; Murphy, P.A.; Hendrich, S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J. Nutr. 1995, 125, 2307–2315.
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584.
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S.
- Lampe, J.W.; Karr, S.C.; Hutchins, A.M.; Slavin, J.L. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc. Soc. Exp. Biol. Med. 1998, 217, 335–339.
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. (Maywood) 2005, 230, 155–170.
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.; Adlercreutz, H.; Bowey, E.A. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 2000, 36, 27–32.
- Watanabe, S.; Yamaguchi, M.; Sobue, T.; Takahashi, T.; Miura, T.; Arai, Y.; Mazur, W.; Wähälä, K.; Adlercreutz, H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J. Nutr. 1998, 128, 1710–1715.
- Arai, Y.; Uehara, M.; Sato, Y.; Kimira, M.; Eboshida, A.; Adlercreutz, H.; Watanabe, S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J. Epidemiol. 2000, 10, 127–135.
- Song, K.B.; Atkinson, C.; Frankenfeld, C.L.; Jokela, T.; Wähälä, K.; Thomas, W.K.; Lampe, J.W. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006, 136, 1347–1351.
- Setchell, K.D.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193.
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. (Oxf.) 2013, 78, 365–372.
- Yoshikata, R.; Myint, K.Z.; Ohta, H. Relationship between equol producer status and metabolic parameters in 743 Japanese women: Equol producer status is associated with antiatherosclerotic conditions in women around menopause and early postmenopause. Menopause 2017, 24, 216–224.
- Kawano, K.; Hirashima, T.; Mori, S.; Saitoh, Y.; Kurosumi, M.; Natori, T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 1992, 41, 1422–1428.
- Wanezaki, S.; Saito, S.; Inoue, N.; Tachibana, N.; Shirouchi, B.; Sato, M.; Yanagita, T.; Nagao, K. Soy β-Conglycinin Peptide Attenuates Obesity and Lipid Abnormalities in Obese Model OLETF Rats. J. Oleo Sci. 2020, 69, 495–502.
