Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Feline polycystic kidney disease is a disease with high prevalence in some feline breeds such as the Persian breed. This disease is characterized by chronic renal failure, appears in animals between three and ten years of age and leads to severe and irreversible kidney failure.
- control disease
- feline polycystic kidney disease
1. Introduction
Polycystic kidney disease (PKD) is an inherited disease that causes a progressive development of fluid-filled cysts in the kidney and, sometimes, in other organs such as liver and pancreas [1]. Cyst formation and growth progress slowly, causing deterioration of kidney tissue and a gradual decrease in kidney function, leading to irreversible kidney failure. This hereditary pathology of autosomal dominant transmission represents one of the genetic diseases with the highest prevalence in humans, where it is called Autosomal Dominant Polycystic Kidney Disease (ADPKD). ADPKD affects from 1:200 to 1:1000 people [2]. In cats, this disease also has a high prevalence, mainly in the Persian breed, being in this breed one of the most prevalent feline genetic diseases, along with diabetes and feline lower urinary tract disease [3,4,5]. However, the Persian breed is not the only breed affected by this disease. Other breeds such as the Exotic Shorthair, Himalayan, British Shorthair, American Shorthair, Burmilla, Ragdoll, Maine Coon, Neva Masquerade and Chartreaux breeds can be affected by this pathology [3,6,7,8,9,10]. Currently, imaging tests such as ultrasound seem to be reliable methods in the diagnosis and monitoring of the disease [4,11,12]. Additionally, multiple genetic tests have been developed to determine the presence of the responsible mutation, giving breeders, owners and clinics the ability to easily detect PKD at an early stage [4,8,13]. Thus, these early diagnosis techniques would allow the establishment of selection programs to reduce or eliminate this pathology in cats.
2. Epidemiology of Feline PKD
The first study that analyzes the prevalence was carried out in the United States, where the prevalence of PKD in Persian cats was around 38% [11]. Later, other authors studied the prevalence in this breed in Australia (50%), the United Kingdom (49.2%), France (40.45%), Italy (41%), Slovenia (36%), Taiwan (15.7%), Iran (36.38%), Japan (46%) and Brazil (5%) [6,12,13,14,15,16,17,18,19]. In these studies, no statistically significant differences were documented between males and females, suggesting that the inheritance of the disease is not sex-linked [14,20]. It has been studied less in other feline breeds such as Neva Masquerade cats or Siberian cats [9,21], although some authors suggest that it could be present in all feline breeds, since around 80% of all current feline breeds have had some type of cross with the Persian breed, so they could have inherited the mutation that provokes the disease [22]. In fact, Lyons et al. (2004) concluded that PKD affects 6% of the total feline population around the world. This would represent that this pathology is the most prevalent genetic disease in cats [5]. This high prevalence and the lethal nature of the disease justify the increasing interest of veterinarians and breeders in this disease. Thus, in 2001, the English organization Feline Advisory Bureau (FAB), now International Cat Care (ICC), developed a research program with selective purpose in the United Kingdom. The main aim was to identify affected cats, create a registry of animals with the genetic tests, and thus allow breeders to select healthy animals in their breeding lines [23]. More recently, a study in Brazil indicates a significant decrease in feline PKD prevalence (5% in Persian cats), which could be related to the first success of genetic counseling [19]. However, more studies should be carried out to evaluate the repeatability of the data obtained and eliminate possible mistakes. Although this is the first study to analyze the results of genetic counseling in reducing the prevalence of the disease, there is no doubt that good advice from veterinarians in the genetic selection of cats can greatly reduce this severe pathology.
3. Genetic Aspects of Feline PKD
Since the 1970s, several cases of feline PKD have been described in the literature. In 1990, Biller et al. hypothesized for the first time a hereditary nature for the disease, after studying a 6-year-old female Persian cat that had been crossed with a healthy male Persian cat [24]. The female was referred for hematuria and polyuria-polydipsia, whose diagnosis of PKD was made by ultrasound and confirmed by anatomical-pathological examination. This female had given birth to five kittens divided into two litters. Ultrasound examination of four kittens showed that two males and one female were affected by PKD [24]. In 1996, the same authors identified the type of inherited transmission of PKD in a study carried out in a colony of cats. For this study, the authors created an experimental pedigree of affected cats from the case previously studied in 1990 (Figure 1). To create the colony, two litters obtained by crossing this female with a healthy Persian male were used. All affected cats in this family were identified by ultrasounds by anatomical-pathological analysis, or both [25].
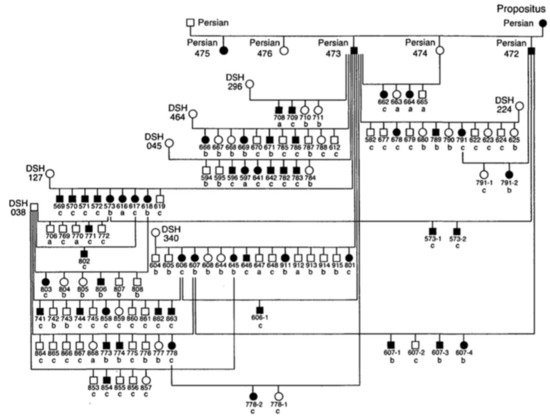
Figure 1. Pedigree of the colony of cats affected by Polycystic kidney disease (PKD). Square = male, circle = female, black symbol = affected cats, white symbol = unaffected cats, DSH = domestic shorthaired cat, Persian = Persian cat, a = renal histology, b = ultrasound, c = renal histology and ultrasound [25].
The results of this study made it possible to identify the autosomal dominant mode of transmission of PKD. The statistical analysis showed proportions that corresponded to an autosomal dominant inheritance with complete penetrance. The animals suffered from the disease only with the presence of a defective allele in their genotype and with a penetrance of 100% (it occurred in all individuals) [25]. To date, no homozygous animals have been found for the mutation of PKD1 gene, which reinforces the idea that it is lethal in utero [5,8,17].
Feline PKD was first identified in 1969 in some sporadic cases [26], and it was described in more detail in the 1990s, with the identification of the hereditary transmission and its similarity to humans ADPKD [25]. The following studies made it possible to discover the implicated gene called PKD1 and the mutation that provokes the disease [5,27]. The PKD1 and PKD2 genes code for the polycystin-1 and 2 proteins. Mutations in these genes are responsible of 85% and 15% of human ADPKD cases, respectively, and affect the kidney and the bile duct [28]. The presence of cysts depends on animal age, so 90% of presumed gene carriers will have cysts that can be identified based on the age of the animal [2,29]. For example, a clinical case was described in six kittens that died at seven weeks of age, in which the necropsy revealed the presence of renal and bile duct cysts, undiagnosed by the young age of the animals [30]. Recent studies in human medicine reveal high genetic and clinical heterogeneity of this disease [31]. Thus, although now only the PKD1 gene has been identified as the cause of the disease in cats, several studies found cases of animals with kidney cysts and a wild-type genotype of the PKD1 gene. This raises the existence of other responsible mutations and, therefore, a possible genetic heterogeneity of feline PKD [3,8,32].
4. Pathophysiology and Clinical Features
Regarding the pathogenesis of the disease, there are still many causes under study, and the pathogenesis processes are not well understood. In humans, abnormalities in gene expression, cell polarity, fluid secretion, and apoptosis have been hypothesized. It seems that the formation of the cysts could be related to a hyperplasia of the tubular epithelium, which causes a partial obstruction of the tubules, preventing the flow of urine [7]. The mutation of the PKD1 gene triggers the modification of the polycystin-1 protein, which is expressed in the primary cilium, a flagellar structure originating from the tubular cell and in contact with the urinary flow. These cilia are organelles that function in fluid transport and chemo and mechanoreceptors [42,43]. Currently, the precise function of polycystin-1 is unknown, but it appears to be involved in cell–cell and matrix–cell interactions [44]. The predominant hypothesis about the pathogenesis of ADPKD focuses on the role of the cilium–centrosome complex of tubular epithelial cells. Disorders that result in defects of this complex are called “ciliopathies” and many of the associated disorders have renal cysts as a part of their pathology [40,44]. The cyst formation process seems to occur through the combination of increased cell proliferation, fluid secretion, and extracellular matrix alterations, so the loss of polarization of the cilia would alter the water reabsorption function, developing cysts in the parenchyma [45].
Feline PKD is characterized by the presence of cysts, in variable number and size, in the renal parenchyma. The cysts are present from birth, they form in the cells of the renal tubules and most of them are observed in the cortex or in the cortico-medullary area [18]. These cysts increase in number and size proportionally with age, which explains that many cats are still subclinical for several years [36]. The clinical signs of PKD are not pathognomonic for this condition, as it manifests as chronic renal failure. The average age of appearance of clinical signs is established at seven years, but they can appear between three and ten years [4,17,26]. In general, the clinical signs observed on the basis of history can be apathy, anorexia, weight loss, bad appearance of the coat, polyuria and polydipsia, as well as gastrointestinal disorders [26,46,47]. On clinical examination, general dehydration, pale mucous membranes can be observed, as well as increased volume and irregular contour of the kidneys on palpation. Although curative treatment does not exist, these clinical signs can be alleviated with palliative treatment.
In affected cats, laboratory findings are not specific, mainly indicating renal failure (azotemia, hyperphosphatemia, non-regenerative anemia, and proteinuria). However, clinical stages can be highly variable, as demonstrated in a recent study where several young animals presented azotemia with a remarkably high creatinine concentration, compared to older animals with less important values [18]. Several authors have found that there is significant individual variation in the progression of disease, although there is still no conclusive evidence [36]. The variability observed between cats, as well as the variability that can occur between the two kidneys of the same cat, suggests that other factors can change the expression and progression of the disease. Clinically relevant aspects include renal manifestations, but there are also extrarenal manifestations where liver involvement is the most common. The hepatic cyst is an extrarenal manifestation that occurs in some cases of feline PKD [38]. In humans and cats, the rate of matching liver and kidney cysts is approximately 80% and 12.6%, respectively [36,48]. However, in humans there is also a marked dilatation of the bile ducts associated with cysts, while cats do not show other hepatobiliary lesions, so liver cysts could have a different pathogenesis from humans ADPKD [38]. Furthermore, there were no statistically significant differences between the age of the cat and the stage of disease with the presence of liver cysts. The stage of the disease could not be related to the formation of liver cysts, although some studies reported cases of related liver fibrosis in cats with PKD [36,38]. Nowadays, clinical signs associated with liver failure have rarely been found, and the inherited nature of this process has not been established.
In cats, mutated polycystin-1 seems to play a significant role in cell proliferation and differentiation of the tubular epithelium, in addition to known antiapoptotic activity [49]. Thus, the balance between tubular degeneration, activation of necrosis and apoptosis is a key factor in the appearance of cysts. In this way, the induction of cell death in affected cells could be related to the pathogenesis of the disease [41]. In addition to cystic structures, fibrosis of kidney tissue and increased expression of transforming growth factor beta (TGF-β) around these fibrous areas were observed, suggesting that in animals with PKD, renal failure may also be caused not only by cyst formation but renal fibrosis could be a crucial factor [41,49]. Another factor that must be considered is that there are other mutations that cause ciliopathies, as well as other diseases that can generate kidney cysts [50]. These pathologies can mimic PKD and should be considered as phenocopies when studying the mechanisms of PKD [46]. To date, the etiopathology of the disease is not defined and is based on different hypotheses. It is being studied in both humans and veterinary medicine to explore the differential formation of kidney cysts.
5. Diagnosis of Feline PKD
Diagnosis of PKD cannot be established by the only clinical features. For example, renal palpation can reveal nephromegaly, but this can be caused by other pathologies. The previously mentioned clinical signs, the evidence of renal failure by laboratory findings and epidemiological data (mainly, feline breed) can guide the diagnosis of disease [14]. However, current methods of choice are imaging tests, mainly ultrasound, and recently developed genetic study methods.
5.1. Imaging Diagnostic
The use of imaging tests is essential in the feline PKD diagnosis. Radiography and intravenous urography can be used in more advanced cases, when there are multiple, large cysts. However, the examination with the most success is that of ultrasound, which allows a quick and reliable diagnosis to be obtained, and it is the only current method that decides the severity and progression of the disease [36].
5.2. Genetic and Molecular Diagnosis
Based on the identification of the gene involved in the development of PKD [5], several methods have been established for the identification of the mutation responsible for the disease. The PCR method is mainly used to identify and amplify the DNA fragment of interest. There are different variants of PCR that have been used and confirmed in different studies.
This entry is adapted from the peer-reviewed paper 10.3390/vetsci8110269
This entry is offline, you can click here to edit this entry!
