Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Biomineralization is a process in which organic matter and inorganic matter combine with each other under the regulation of living organisms.
- Mineralization
- Biomineralization
- Temperature
- biological control
1. The Type of Mineralization
According to the degree of biological mineral control, biomineralization could be divided into two categories: biological induction and biological control. Bio-induced biomineralization is a process caused by physiological activities of organisms, such as metabolism, inhalation of oxygen, exhalation of carbon dioxide by respiration, as well as establishment of cell walls, which change the physical and chemical conditions of the surrounding environments. This mineralization is not guided by specific cell tissues or biological macromolecules, resulting in arbitrary orientation of crystals and lack of unique morphology. Negatively charged cell walls (containing carboxyl and phosphatidyl groups) combine Fe3+ ions by electrostatic interaction, and Fe3+ ions react with silicic acid to form iron silicate. This process is seldom controlled by cells, and its crystalline form is similar to that of iron silicate that is produced in inorganic solutions. Biologically controlled mineralization is a process that is caused by biological physiological activities and controlled by biology in three aspects: space, structure, and chemistry. It occurs in delineated confined spaces. Biomineral organic matter is formed with high content, unique crystallization habit, uniform size, uniform shape, and regular arrangement.
There are two forms of mineralization: one is normal mineralization, such as skeleton, teeth and shellfish, formation of shells, and so on. Another is abnormal mineralization, such as stones, dental stones and caries teeth, and so on. There are two theories to explain this biomineralization. Solution crystallization theory and polymer-induced liquid phase precursor mineralization theory. For solution crystallization theory, Type I collagen molecules self-assemble into a native “hole”, the “hole” contain negatively charged amino acids, the –COO– could bind to Ca2+, which lead to the deposition of Ca2+ in the “hole”[15]. After deposition, the calcium phosphate (Ca-Pi) is electrostatically formed through binding. The Ca–Pi nucleus is the basic building blocks of bone structure [16]. In addition, the “hole” is the nucleation site of hydroxyapatite crystals, which is another important component of bones [17]. For polymer-induced liquid phase precursor mineralization theory, highly hydrated amorphous calcium phosphate phase nanometer droplets penetrate into the pores and voids of collagen fibers the hydrates lose water and crystallize in the pores and voids of collagen fibers [18]. The calcification of atherosclerosis (AS) plaque is a typical sample of abnormal mineralization.
2. Factors Controlling Biomineralization
Mineralization in organisms can be divided into four stages. (1) Organic macromolecules are pre-assembled into ordered structures. Insoluble organic matter in organisms constructs an organized micro-reaction environment before mineral deposition, which determines the location of inorganic nucleation and the function of mineral formation. This stage is the precondition of biomineralization. (2) Molecular recognition of organic-inorganic interfaces controlling crystal nucleation and growth. (3) Growth regulation enabling the initial assembly of crystals to form subunits. (4) Cell prepressing, forming biominerals with multilevel structure by assembling subunit minerals. It is believed that biomineralization is controlled by varying factors. The formation of biominerals is often the result of the synergistic action of various factors, including pH, temperature, and matrix.
2.1. Temperature and pH
Temperature is an important factor affecting calcium carbonate deposition. The solubility of most salts in water will increase with the increase of temperature. However, calcium carbonate has abnormal solubility, the solubility will decrease when the temperature rises. That is to say, more calcium carbonate will deposit when the temperature rise. pH also has great influence on the solubility of carbonate. Reducing PH value will increase the solubility of carbonate. Furthermore, pH and temperatures can control calcium carbonate particles forming various crystal morphologies [19], such as, plate, rhombohedra, rectangles ellipsoid, cube, etc. Vaterite with specific morphologies was formed at low temperature, whereas needle-shaped aragonite crystals were obtained at high temperature [20,21].
There exists some controversy pertaining about the temperature effect on the biomineralization forming process. Some previous studies on the oysters C. gigas and C. virginica have found that raising temperature has stronger effects than moderate ocean acid on shell growth and metabolism [22,23]. The lesser pH effect demonstrated here agrees with D.G.H., et al. [24].
pH is the most important factor affecting the precipitation structure in Uranium (VI) biomineralization by Saccharomyces cerevisiae [25]. Some other reports have shown that there were no negative effects on the calcification rate, CaCO3 deposition, shell ultrastructure, and metabolic pathways in P. fucata exposed to warmed seawater [26]. If the thermal threshold is not breached, temperature elevation may promote CaCO3 deposition during biomineralization [26], as shown in Figure 1(A). Indeed, the effects of temperature rise on organic biomineralization vary from species to species. The effects of temperature and pH value on biomineralization are synergistic. It was also recognized that the Mg content in coralline carbonate varied seasonally experiments on synthetic Mg-calcite [27], which demonstrated a positive correlation between Mg content and temperature. Sometimes, they would need to improve the thermostability. It should be noted that egg cells with mineral shells are extremely thermostable. The stability of modified minerals could also be maintained at suitable pH [28] (Figure 1(B)) and temperature [29] (Figure 1(C)).
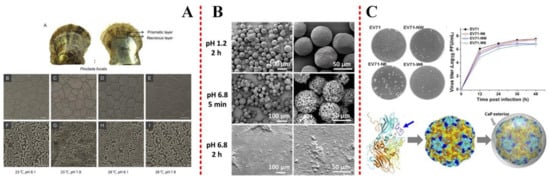
Figure 1. (A) Scanning electron micrographs of Pinctada fucata shells exposed to seawater acidification and elevated temperatures. Reprinted with permission from Ref. [26] Copyright (2016) American Chemical Society. (B) The coating of nanoparticles (NPs) with chitosan (CS) significantly enhanced their stability in intestinal pH. Reproduced by permission of Wiley-VCH from Ref. [28]. (C) Homology modeling of the mutant viral protein. EV71 capsid proteins VP1, VP2, and VP3 are shown in cyan, yellow, and orange, respectively. Reproduced by permission of National Academy of Science from Ref. [29].
pH plays a significant role in controlling pores diameter on the surface of virus, which is usually used as an environment for biomimetic mineralization. The nano-in-micro composites were produced by coating oxidation-responsive NPs with chitosan and encapsulated in a pH-sensitive polymer. The microparticles maintain integrity at acidic pH, which could be used in drug release. The detrimental effects of ocean acid, pH altering, on biomineralization are a common response of marine organisms to environmental changes [30,31,32].
2.2. Guidance of Organic Matrix on Biominerals
The organic matrix in biominerals can be defined as any organic matrix localized at the surfaces of constituents, such as proteins, phospholipids, collagen and carbohydrates, and so on, which act as a mediator for the mineralization of biological systems. A large number of organic matrices, especially proteins, are involved in the formation of inorganic materials in the process of biomineralization, controlling the nucleation [33,34], growth, crystalline form, and trend of inorganic crystals. This process is also called molecular recognition. The macromolecules also play a role in initiating nucleation and directing crystal growth.
2.3. Additives
In recent years, the chemical composition and complex of additives have tremendously attracted scientific attention. Detailed controlling mechanism of additives on mineralization could be reviewed in recent Denis Gebauer’s paper [35]. Additives and matrix could lead to the crystal morphology modification. Insoluble and soluble organic additives provide a heterogeneous nucleation site and regulate crystal growth by their adsorption. Soluble organic substances distribution and incorporation level in the CaCO3 crystals can alter the growth kinetics and morphology of calcium carbonate. Insoluble matrices, as biomacromolecules, can form a three-dimensional framework for promoting the nucleation of CaCO3 crystals and thus lead to a finer crystallization of CaCO3 [36,37]. The inorganic ions have been demonstrated to be incorporated into the lattice of calcite, thus influencing the morphology of calcite crystals [38]. Citrate coated Au nanoparticles (CIT-Au NPs) and agarose gels were both introduced into the crystallization [39]. CIT, and the matrix, agarose gel, have synergistic effects on morphology regulation of synthetic calcite single-crystals. The agarose gel matrix is inert to the crystal morphology in the sense that the agarose gel grown calcite crystals maintain in characteristic rhombohedra. In contrast, CIT additives are active in crystal morphology modification and crystals begin to exhibit curved rough surfaces when grown in solutions. Apart from inorganic ions, the organic components, including amino acids, peptides, diblock copolymers, and proteins, which also contributed to shaping crystals while acquiring an in-depth understanding of crystal-additive interactions.
Polyacrylic acid (PAA) additives can control the morphologies of calcium carbonate crystal at a concentration dependency manner [19]. In the presence of cetyltrimethylammonium bromide (CTAB), various unusual calcium carbonate crystal morphologies, such as dendrite-shaped, flower-like, wheatgrass-like, needle-like, whiskers, and double-taper-like, can be obtained depending on the addition of various organic additives, such as glycol, glycerine, formaldehyde, acetaldehyde, glycol-methyl ether, and glycol-ethylether [40]. Calcite crystals gradually recovered their sharp rhombohedral morphology in the presence of Water soluble matrix (WSM) from irregular state [20]. One-dimensional Ca-deoxycholate fibers, as a novel insoluble organic polymorph controller, was demonstrated to be the key role in mediating the crystallization and controlling self-assembly processes of calcium carbonate [21]. A novel matrix protein Hic31 from the prismatic layer of Hyriopsis Cumingii displaying a collagen-like structure may play important roles in biomineralization of the pearl prismatic layer [41].
In summary, as shown in Figure 2, biomineralization could be divided into two types, normal mineralization and abnormal mineralization, and the forming process mainly regulated by the temperature, pH and additives.
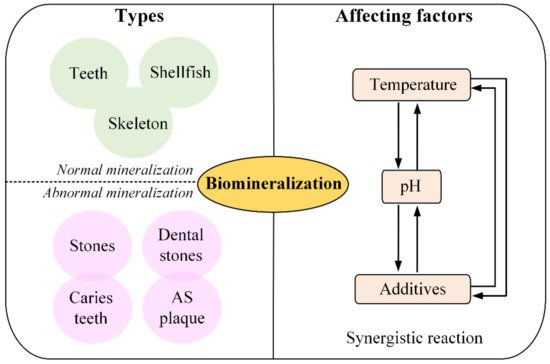
Figure 2. Schematics of types and affecting factors of biomineralization forming process.
3. Mechanism and Approach of Mineralization
The mineralization process in organisms is very complex. Recognizing the formation mechanism of biominerals and matrix, cell and other symbiosis regulation of minerals and substances will be very helpful. Most of the research simply established simulation system and coordinated the regulation of biological macromolecules outside of the organism. There are very few studies on the control effect. Accordingly, we need to be closer to organisms. Further study on the specific regulation of biological macromolecules under environmental conditions function and synergistic regulation to further study organisms in matrix mineralization processes, cell-mineral interactions, to elucidate biominerals.
A deep molecular-level mechanistic understanding of how proteins participate in the nucleation and growth of inorganic crystals (both in vitro and in vivo) can be achieved by computational methods at the atomic scale, as shown in Figure 3(A) [42].
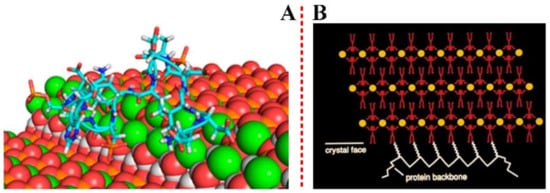
Figure 3. (A) Crystals and proteins have structured surfaces that may contact each other by means of multiple cooperative interactions. Reprinted with permission from Ref. [42] Copyright (2018) American Chemical Society. (B) The protein chain is folded into a secondary structure, often characterized by two and three-dimensional regularity, such as in b-strands and a-helices. Reprinted with permission of Springer from Ref. [43] Copyright (2001).
Michael S. Pacella et, al. compared the results of the Rosetta-Surface algorithm to an experimental benchmark of kinetic and thermodynamic measurements on peptide−biomineral interactions taken from atomic force microscopy, and successfully identified which mineral face and step edges will bind peptides the strongest [42]. The interaction effect between proteins and crystal can be illustrated using examples from biomineralization, as well as by the immune responses from pathological crystallizations to crystalline antigens, as shown in Figure 3(B). A.J., et al. [44] have employed self-assembled monolayers (SAMs) on Au or Ag, with disordered head groups, to induce the formation of an amorphous CaCO3 film. The mechanism of the molecular interactions occurred at the interface between the inorganic mineral and the macromolecules [45]. A form of additive manufacturing, selective laser sintering (SLS) uses a roller feed system, a heated chamber, and a laser to fuse plastic, ceramic powder layers or metal, together form a wide variety of solid objects [46]. As opposed to other printing methods that restrict model geometry or require removable, unfused powder provides support for parts. Together with the biocompatibility of the material feedstocks, this process also gives printed objects a rough surface of partially fused powder and provides a viable avenue for fabricating a wide variety of effective high-surface area substrates for tissue engineering and biomedical implants. Furthermore, the model of three-dimensional printed frameworks has tremendous potential for synthesis of biomaterials. It is especially effective when combined with post-synthesis chemical treatment, and this will be discussed in the later section.
4. Biomimetic Biomineralization
Traditional biomineralization studies emphasize the bionic design and preparation of materials by imitating nature, highlighting the regulation of inorganic crystallization by organic systems, thus improving the properties of materials. The new trend of biomineralization research is to use natural strategies for realizing the regulation of biological organisms through inorganic materials, highlighting the use of material systems to achieve bio-functional transformation.
In 2008, Tang Ruikang’s team successfully realized the mineralization transformation of yeast cells [47], and proposed that using the biomineralization strategy for more biological species could be endowed with new functions through material shells. For example, the preservation of vaccines is highly dependent on the cold chain. Through biomimetic mineralization, the team constructed a new vaccine-calcium phosphate complex, which greatly enhanced the thermal stability of vaccines on the basis of maintaining the original efficacy, and initially realized the construction of heat-stable vaccines independent of refrigerators. Biomimetic mineralization can not only enhance the structural stability of organisms through materials, but also change the original functions of organisms. By inducing the spontaneous accumulation of green algae through biomimetic mineralization of green algae, the group can activate hydrogenase while also ensuring the activity of photosynthetic system II, thus changing the photosynthetic pathway of green algae, and directly decompose water to produce hydrogen under natural conditions. Its hydrogen production efficiency is equal to the normal photosynthetic efficiencies.
When compared with natural or synthetic minerals, biominerals often exhibit excellent mechanical and other properties. The reason is that biominerals have multilevel ordered micro- and nano-structures with specific morphologies, which are also consistent with the macro-scale structure. The acquisition of multilevel ordered mineral structure depends on the synergistic effect of various biomass macromolecules in the process of biomineralization. Clearly, understanding this synergistic effect can better guide material chemists to make controllable composites to obtain excellent mechanical and other properties. However, it is difficult to observe the biomineralization process in situ, and the information that is provided by static biomineral microstructural analysis is relatively limited. Therefore, controllable biomimetic mineralization is still a major problem in the field of material synthesis. Up to now, biomimetic mineralization research is mostly based on empirical knowledge, and biomimetic mineralization research based on controllable route design is few and far between. Calcium carbonate films with shellfish-like prism structure were synthesized by the total synthesis method for the first time, as shown in Figure 4(A), and precise control of the micro-structure of bionic films was achieved, resulting in excellent mechanical properties [48].
Figure 4. (A) Synthetic approach to prismatic-type thin films and their structural similarity to biogenic counterparts [48]. This work is licensed under a Creative Commons Attribution 4.0 International License. (B) The formation mechanism of calcium oxalate needle crystals induced by self-assembled protein nanowire template. Reprinted with permission from Ref. [49] Copyright (2014) American Chemical Society.
Calcium oxalate needle crystals are formed plants using protein nanowires (14 kDa) embedded in needle crystals as templates in banana. Calcium oxalate nanospheres are induced to deposit orderly along the template and they eventually form hexagonal pyramidal mesoscopic structures driven by organic-inorganic self-assembly [49]. This study effectively reveals the structure-functional relationship between the chemical evolution of plant crystal morphology and the environment during the long evolutionary process, as shown in Figure 4(B). That is, in the physiological micro-environment of excessive calcium or oxalic acid metabolism (C4 plants), plants can maximize the capture of calcium and oxalic acid in the limited all oocyte, thus maintaining the metabolism of inorganic calcium and organic acid in plants. It is also of great significance to the development of bionic materials and the study of human pathological mineralization, such as kidney stones (calcium oxalate is also the main component).
Biomineralization, as a functional strategy in the process of biological evolution, can make organisms more adaptable to the environment and they produce evolutionary chains that are more conducive to their own development. It also provides a reference direction for human beings to realize the regulation of biological organisms through materials. By learning from nature, the study of biomineralization has realized the transformation from the regulation of material crystallization by biological system to the improvement of organisms by using materials, which provides a new direction for the sustainable development of human beings.
A rapid synthesis method of multifunctional macro-graphene composites was developed. Graphene oxide was added into biomimetic mineralized gel system to form graphene oxide, amorphous calcium carbonate nanoparticles, and polyacrylic acid cross-linked network structure. The soft and hard state of the graphene composite can be controlled by moisture content. It is also found that the material has excellent plasticity and self-healing ability, and it is expected to be used in multi-scale rapid processing and forming of graphene materials [50].
This entry is adapted from the peer-reviewed paper 10.3390/min9020068
This entry is offline, you can click here to edit this entry!
