Hepatocellular carcinoma (HCC) is one of the most common and lethal cancers worldwide. Currently, treatments available for advanced HCC provide dismal chances of survival, thus there is an urgent need to develop more effective therapeutic strategies. While much of the focus of recent decades has been on targeting malignant cells, promising results have emerged from targeting the tumour microenvironment (TME). The extracellular matrix (ECM) is the main non-cellular component of the TME and it profoundly changes during tumorigenesis to promote the growth and survival of malignant cells.
- extracellular matrix
- liver cancer
- tumour microenvironment
- bioengineering
1. Introduction
Liver cancer is the sixth most common form of cancer in incidence worldwide across both sexes and all ages and in 2020 there were 900,000 cases worldwide. It is the third in cancer-related deaths, claiming more than 800,000 lives globally in 2020 [1]. With incidence on the rise worldwide, it is estimated that by 2030 over 1 million people will die from liver cancer [2]. The most common form of primary liver cancer is hepatocellular carcinoma (HCC) that represents 90% of cases [3]. The survival rate for HCC is poor, with a 5-year rate standing at 18% [4]. Moreover, 90% of HCC cases develop on the back of persistent liver inflammation which could result in aberrant chromosomal changes and can lead to the malignant transformation of hepatocytes [5,6]. The most important risk factor for HCC is cirrhosis as one in three cirrhotic patients will develop HCC during the course of their lives [7]. Other prevalent risk factors for HCC are hepatitis B (HBV) or hepatitis (HCV) infections, excessive alcohol consumption, obesity-related or diabetes non-alcoholic steatohepatitis (NASH), aflatoxin B1, and these risk factors vary by geographical region [3].
HCC is a molecularly highly heterogeneous malignancy and this aspect is present at three levels: interpatient heterogeneity, intertumoural heterogeneity (variability within the tumour nodules of the same patient) and intratumoural heterogeneity (variability between different regions of the same tumour nodule) [8]. This high heterogeneity coupled with the suppressive tumour microenvironment [9] makes creating a universally effective treatment challenging. Currently, treatment is determined by scoring on the Barcelona Clinic Liver Cancer algorithm. Stage 0 and A patients are eligible for surgical resection, however, 70% of patients undergoing resection will have a recurrence within 5 years [10]. Patients with stages B (intermediate) and C (advanced) HCC have systemic therapies available that mainly consists of various multi-kinase inhibitors (e.g., sorafenib, lenvatinib) [11]. Immune checkpoint inhibitor (ICI) monotherapy of cytotoxic lymphocyte antigen-4 (CTLA-4) or programmed cell death (PD-1)/programmed cell death ligand-1 (PD-L1) showed promising results in early clinical trials [12,13,14,15] which resulted in the FDA granting approval to pembrolizumab and nivolumab, now recommended as 3rd line of treatment [11]. However, median overall survival (OS) and objective response rates (ORR) were 1 year and 15% for PD-1/PD-L1 blockade in patients previously treated with sorafenib [12,13], whereas for CTLA-4 blockade the median time to progression was 6.48 months and the ORR was 17.6% [15]. In addition, further randomised trials for anti-PD-1 monotherapy for HCC did not show statistically increased OS either as a first-line treatment (against sorafenib) [16] or as a second-line treatment (against placebo) [17]. However, combination therapy of ICI and other agents have shown promising results. In a global phase 3 clinical trial, atezolizumab (PD-1 inhibitor) was administered alongside bevacizumab (VEGF inhibitor) to patients with unresectable HCC and the combination therapy had better OS at 12 months (67.2% vs. 54.6%) and median progression-free survival (6.8 vs. 4.3 months) than patients given sorafenib only [18]. Other combinations include pembrolizumab (PD-1 inhibitor) and lenvatinib (multi kinase inhibitor) [19], atezolizumab and cabozantinib (multi-kinase inhibitor) [20] or the combination of different ICIs such as durvalumab and tremelimumab [21] and nivolumab and ipilimumab [22].
2. The Extracellular Matrix in the Tumour Microenvironment
The positive effect of immunotherapy, when combined with kinase inhibitors, highlights the importance of the microenvironment in HCC. The tumour microenvironment (TME) in solid tumours is made up of the tumour cells and tumour-associated stroma [23]. The tumour stroma comprises cellular components such as blood and lymphatic vessels, cancer-associated fibroblasts (CAFs) and immune cells, as well as non-cellular components such as the extracellular matrix (ECM) [23]. While much valuable information has been extracted from 2D cell culture models of HCC, they fail to account for various important environmental factors that can affect HCC progression and therapy. One such factor is the change in composition and mechanical properties of the ECM.
The typical remodelling of the ECM that occurs in cancer development and growth serves as a supportive environment for tumorigenesis, the maintenance of cancerous tissue and metastasis. ECM remodelling is driven by various processes in cancer [35], summarized in Table 1 . Commonly, there is an accumulation of ECM components such as collagens in the tumour stroma [36]. Ma et al. demonstrated that COL1A1 is highly expressed in HCC and can be used as a putative biomarker for HCC carcinogenesis and metastasis [37]. In addition, it has been shown that HSCs trigger the epithelial to mesenchymal transition (EMT) of hepatocellular carcinoma cells via the secretion of type I collagen [38]. Overtly abundant collagen can also increase cancer cell survival. By binding to integrins, it increases focal adhesions, initiates PI3K signalling and promotes the growth of tumour cells [39].
Table 1. Main ECM remodelling processes in cancer in respect to normal ECM.
| Increased ECM Deposition | ECM Degradation | Altered Post-Translational Modifications |
|---|---|---|
| ↑Fibronectin, collagens I, III, IV | ↑ECM degradation ↑Creation of bioactive molecules (matrikines) ↑Release of growth factors ↑Angiogenesis ↑ Metastasis |
↑LOX activity ↑ECM stiffness ↑Mechanosignalling ↑Interstitial fluid pressure ↓ Impeded immune cell activity |
The ECM includes proteins that have a variety of effects on the immune system and there is increasing evidence pointing to the role that ECM proteins play in immune exclusion. As a result of increased density, it plays a crucial role in T cell exclusion. In fresh, human lung ex vivo tumour slices, T-cells preferentially accumulated in the stroma with 5 times more T-cells there than in the tumour [90]. T cells were able to migrate better in looser collagen and fibronectin regions and collagenase treatment reversed the obstructing effects of the tumour stroma on T-cell migration [90]. Moreover, T-cells are known to migrate by reorganising their cytoskeleton that results in considerable cellular deformations allowing them to migrate through narrow spaces. However, when they are confronted by dense ECM they are unable to migrate through them and as a result, they migrate away towards looser ECM [91]. Treatments that target the components of the ECM could help: for example, collagenase treatment has been shown to increase T cells and tumour cells interactions [90]. Tenascin-c, a glycoprotein whose level increases in the tumour ECM, also helps trap T-cells in the stroma by binding to Toll-like receptor 4 (TLR-4) and inducing the upregulation of CXCL12 expression [92]. In a mouse pancreatic ductal adenocarcinoma (PDAC) model, antifibrotic treatment reduced hyaluronan concentration allowing a greater infiltration of immune cells into the tumour [93]. In pancreatic cancer, T cells accumulated in low collagen density areas and their ability to invade the tumour was inversely proportional to the density of the collagen matrix [94]. Moreover, a dense collagen network was able to abrogate chemokine-induced T-cell migration as well [94]. In a 3D model, high collagen density reduced T-cell proliferation, promoted CD4+ T cells over CD8+ T cells and reduced cytotoxic activity [95].
Treatments that target the components of the ECM could help: for example, collagenase treatment has been shown to increase T cells and tumour cells interactions [90]. A study has also shown that non-response to the anti-PD-L1 antibody atezolizumab in a cohort of metastatic urothelial cancer patients was correlated with the exclusion of CD8+ T cells from the tumour and their accumulation in the tumour stroma [101].
3. Models to Study the Cellular and Non-Cellular Components of the TME
Two-dimensional (2D) cell cultures include primary cells and immortalised cell lines and while primary cells are valuable because they retain donor-specific features, their use is limited by slow growth and a short lifetime. Immortalised cell lines on the other hand can multiply indefinitely, but this makes them less representative of the original tumour. Moreover, the longer these cells are passaged the higher the chances of genetical and phenotypical changes that can affect results [111]. While 2D culturing techniques serve as a cost-effective way of testing compounds, they cannot reconstitute the heterogeneity of the tumour or the complexity of the extracellular environment [111] and this can hinder successful drug development. Three-dimensional models confer various advantages over conventional 2D culturing methods and these are key factors to explain why drugs show a different response in a 3D culture environment. Two-dimensional cultures lack the same morphological organisation as 3D cultures [112]. The spatial organisation of cell surface receptors is different to cells cultured in 3D and this could affect drug binding efficiency [113]. Cancer cells cultured in 3D better recapitulate the in vivo environment where cells are in various different stages of their life cycle [113]. It has been shown that tumour cells de-differentiate in 2D culture whereas in 3D they resemble closer in vivo morphologies [114,115]; cancer cells in spheroid cultures display increased angiogenic factors compared to cells in 2D cultures [116] and that cells cultured in 2D have lower IC50 values for drug treatment than cells cultured in a 3D environment [117]. Recently, a study analysed the metabolome of mouse inner medullary collecting duct cell line (mIMCD3) grown as spheroids and compared it with the metabolome of freshly isolated cells from the mouse distal ducts [118]. They found that the metabolome of cells grown in spheroids was analogous to freshly isolated cells and that cells grown in a 2D environment had a vastly differing metabolome. Signalling is also different in a 3D environment. Head and neck cancer cells displayed upregulation of CDH1, Nanog and Sox2 when cultured in spheroids compared to when cultured in 2D [119]. Colon cancer cells have also shown a downregulation of AKT, mTOR and S6K signalling in spheroid culture compared to 2D culturing [120].
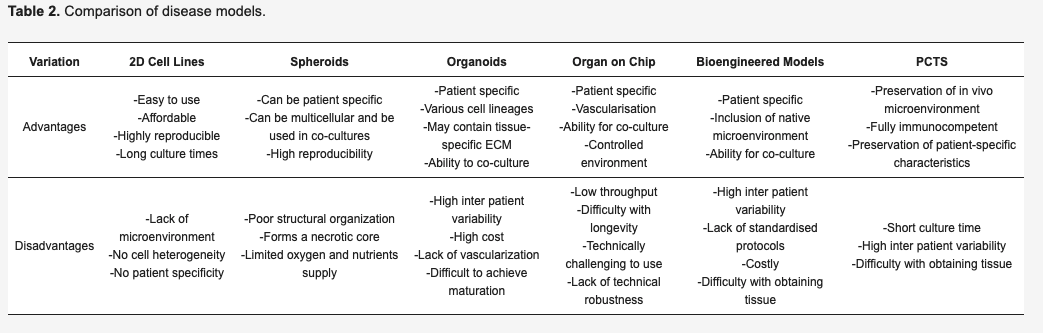
Since 3D cultures are more representative of the in vivo environment, they represent better systems to model cell–ECM and cell–cell interactions and their use is becoming more frequent. Currently, the most developed models of cancer that introduce the presence of a TME are represented by organoids, spheroids, organ-on-chips, precision-cut tissue slices and bioengineered tumour models, reported in Table 2.
4. Conclusions
With rising liver cancer incidence worldwide, the need for new, efficacious treatments that are particularly effective in advanced cases is ever increasing. The success of approaches that combine immunotherapy with the targeting of the TME has highlighted the importance of considering the microenvironment during drug development. TME is an intercellular space that participates in various aspects of tumorigenesis including sustained proliferative signalling, angiogenesis, metastasis [65]. The TME is formed by both cellular and non-cellular components. The importance of a number of proteins of the ECM has been increasingly appreciated in driving tumorigenesis in various human carcinomas. The interplay between immune cells and ECM in the HCC TME is a key aspect that should be studied in respect to disease progression and therefore taken more into consideration in cancer models.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13215586
