Restless legs syndrome (RLS) is a sleep-related movement disorder characterized by an unpleasant urge to move the lower limbs. The prevalence of RLS varies by region, ethnicity, sex, and age, ranging from 5–15% . Its pathophysiology remains unclear. Criteria for the diagnosis of RLS include the International Restless Legs Syndrome Study Group (IRLSSG) and International Classification of Sleep Disorders, Third Edition (ICSD-3). The ICSD-3 criteria require distress and associated sleep disturbance, which is different from the IRLSSG consensus [2]. As for the measurement of disease severity for RLS, the IRLSSG rating scale (IRLS) was proposed. It assesses a range of RLS related symptoms and their impact on patients’ mood and daily life, and it has been proved reliable, valid, and responsive in clinical trials.
- botulinum toxin
- restless legs syndrome
- systematic review
- meta-analysis
1. Introduction
2. Results of Quantitative Synthesis
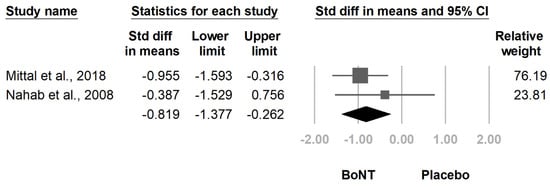
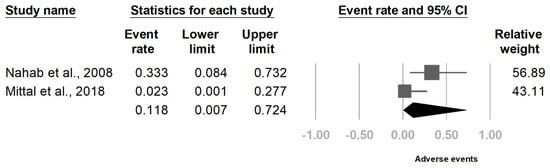
3. Current Insights
This entry is adapted from the peer-reviewed paper 10.3390/healthcare9111538
References
- Yeh, P.; Walters, A.S.; Tsuang, J.W. Restless legs syndrome: A comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012, 16, 987–1007.
- Sateia, M. International Classification of Sleep Disorders-Third Edition. Chest 2014, 146, 1387–1394.
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014, 15, 860–873.
- Abetz, L.; Arbuckle, R.; Allen, R.P.; Garcia-Borreguero, D.; Hening, W.; Walters, A.S.; Mavraki, E.; Kirsch, J.M. The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical-trial setting. Sleep Med. 2006, 7, 340–349.
- Wilt, T.J.; MacDonald, R.; Ouellette, J.; Khawaja, I.S.; Rutks, I.; Butler, M.; Fink, H.A. Pharmacologic Therapy for Primary Restless Legs Syndrome: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2013, 173, 496–505.
- Harrison, E.G.; Keating, J.L.; Morgan, P.E. Non-pharmacological interventions for restless legs syndrome: A systematic review of randomised controlled trials. Disabil. Rehabil. 2019, 41, 2006–2014.
- Silber, M.H.; Becker, P.M.; Earley, C.; Garcia-Borreguero, D.; Ondo, W.G. Willis-Ekbom Disease Foundation Revised Consensus Statement on the Management of Restless Legs Syndrome. Mayo Clin. Proc. 2013, 88, 977–986.
- Mitterling, T.; Heidbreder, A.; Stefani, A.; Fritz, J.; Ulmer, H.; Poewe, W.; Högl, B. Natural course of restless legs syndrome/Willis-Ekbom disease: Long-term observation of a large clinical cohort. Sleep Med. 2015, 16, 1252–1258.
- Lanza, G.; Bachmann, C.G.; Ghorayeb, I.; Wang, Y.; Ferri, R.; Paulus, W. Central and peripheral nervous system excitability in restless legs syndrome. Sleep Med. 2017, 31, 49–60.
- Stiasny-Kolster, K.; Pfau, D.B.; Oertel, W.H.; Treede, R.D.; Magerl, W. Hyperalgesia and functional sensory loss in restless legs syndrome. Pain 2013, 154, 1457–1463.
- Stiasny-Kolster, K.; Magerl, W.; Oertel, W.H.; Möller, J.C.; Treede, R.D. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain 2004, 127, 773–782.
- Kerr, S.; McKinon, W.; Dafkin, C.; Bentley, A. Characterization of painful Restless Legs Syndrome sensations in an English-speaking South African population. Scand. J. Pain 2019, 19, 483–489.
- Argoff, C.E. A focused review on the use of botulinum toxins for neuropathic pain. Clin. J. Pain 2002, 18, S177–S181.
- Mittal, S.O.; Safarpour, D.; Jabbari, B. Botulinum Toxin Treatment of Neuropathic Pain. Semin. Neurol. 2016, 36, 73–83.
- Park, J.; Chung, M.E. Botulinum Toxin for Central Neuropathic Pain. Toxins 2018, 10, 224.
- Gazerani, P.; Staahl, C.; Drewes, A.M.; Arendt-Nielsen, L. The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain 2006, 122, 315–325.
- Rotenberg, J.S.; Canard, K.; Difazio, M. Successful treatment of recalcitrant restless legs syndrome with botulinum toxin type-A. J. Clin. Sleep Med. 2006, 2, 275–278.
- Agarwal, P.; Sia, C.; Vaish, N.; Roy-Faderman, I. Pilot trial of onabotulinumtoxina (Botox) in moderate to severe restless legs syndrome. Int. J. Neurosci. 2011, 121, 622–625.
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum Toxin in Restless Legs Syndrome-A Randomized Double-Blind Placebo-Controlled Crossover Study. Toxins 2018, 10, 401.
- Richardson, D.; Bajwa, R.; Eisa, M.; Miller, D.; Mohsenin, V.; Jabbari, B. Botulinum toxin a treatment can improve symptoms of Restlesslegs syndrome. Mov. Disord. 2007, 22, S269.
- Ghorayeb, I.; Burbaud, P. Failure of botulinum toxin A to relieve restless legs syndrome. Sleep Med. 2009, 10, 394–395.
- Ghorayeb, I.; Bénard, A.; Vivot, A.; Tison, F.; Burbaud, P. A phase II, open-label, non-comparative study of Botulinum toxin in Restless Legs Syndrome. Sleep Med. 2012, 13, 1313–1316.
- Nahab, F.B.; Peckham, E.L.; Hallett, M. Double-blind, placebo-controlled, pilot trial of botulinum toxin A in restless legs syndrome. Neurology 2008, 71, 950–951.
- Silva, M.A.; Duarte, G.S.; Camara, R.; Rodrigues, F.B.; Fernandes, R.M.; Abreu, D.; Mestre, T.; Costa, J.; Trenkwalder, C.; Ferreira, J.J. Placebo and nocebo responses in restless legs syndrome. A systematic review and meta-analysis. Neurology 2017, 88, 2216–2224.
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins 2016, 8, 65.
